1. Introduction
Some research has claimed that the origin of life has been highly dependent on clays and their changes [1]. The main reasons for the claim are the ordered arrangement and space between crystal layers, and the large adsorption capacity and chemical organic concentration ability, among others. This theory also suggests that the polymerization of amino acids and nucleotides, which are the basic substances of RNA, has been possible through the changes of natural clays that have performed an active role as efficient catalysts [1] and as selectively adsorbed drink extracts [2,3]. Clays have shown natural changes as a result of cation exchanges, temperature, and geological forces. These changes have favored the evolution of clays and their applications over a period of time.
Currently, the applications of clays have continued to be relevant because of their natural origin, high abundance and low cost, among others. Moreover, clays have been subjected to different physical and chemical methods to enhance their performance. Some of these methods are mineral extraction and purification treatments, chemical pillarization using organic and inorganic molecules, thermal treatments, and pressure variation [4-6], and most of the treatments are focused on enhancing the layer space. In this case, the insertion of large molecules into the microstructure of clays or on specific sites is performed. After the treatments, the physical properties of the clays change, and meso- and macro-porous materials can be obtained [7,8].
In Colombia, clays obtained from the northern Tolima region came from volcanic or sedimentary rocks with volcanic contributions of paleogenic and neogenic periods [9, 10], which explained the abundance of smectite. This group of clays is used in different applications because their physical and chemical properties can be modified after chemical treatments. For instance, thermal stability and swelling behavior showed a strong relationship with the differences in the electrical charge of the layers, the origin of the charge deficiency, and interlayer cations [11]. Moreover, these features have motivated the study of Colombian clays in different fields such as environmental remediation, catalysts, and waste treatments [7,8,10,12]. Currently, commercial exploitation is mainly concentrated in animal feeding additives and cat litters.
On the other hand, the ceramic industry usually uses binary, ternary, and multiple mixtures of clays as raw materials to produce different earth-ware products. Usually, 0 w.t% to 100 w.t% of each compound with 20 or 25 w.t% intervals are used for binary mixtures [13]. In addition to the composition, other factors related to processing such as grain size, pressure, mixture concentration and thermal treatment are necessary to understand the technical characteristics and final properties of a ceramic product [14].
The aim of this study is to compare these features using the physical mixtures of two Colombian smectite clays. This study is more focused on the changes in textural and structural properties after thermal treatments than on ceramic characteristics. Reports about natural zeolites [15] and other thermally-treated clays have [16] motivated this study.
2. Materials and methods
2.1. Materials
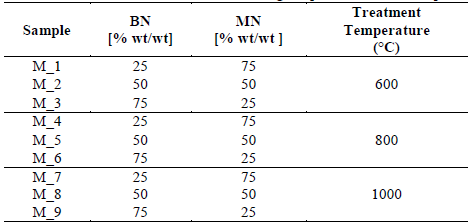
Bentonite (BN) and montmorillonite (MN) samples were obtained from two commercial natural clays (BAST SAS and CIPA SA companies, respectively). Both the Colombian companies focus on animal feeding additives and cat litter products. The raw material was analyzed on the basis of structure and texture. Samples were obtained without any purification process. Both the clay samples where physically dry mixed at three different weight proportions 25 w.t %, 50 w.t %, and 75 w.t %)for 15 min using a closed glass bottle. All samples were crushed and sieved in Tyler No 325, 45 µm to obtain fine powders. Then, they were thermally treated in a conventional furnace at three temperatures (600°C, 800°C, and 1000°C) for 4 h under an air atmosphere and at a heating ramp of 10ºC/min. The experimental variables and the names of the samples are shown in Table 1. The temperatures values were chosen to produce textural changes of the clays and avoid melting and bonding of metallic oxides at temperatures higher than 1000°C.
2.2. Methods
2.2.1. X-ray techniques
X-ray fluorescence (EDXRF) data were obtained using BRUKER S2 PUMA equipment with a Pd X-ray tube.
X-ray diffraction (XRD) was performed in a SIEMENS D 500. The XPERT apparatus was equipped with a graphite monochromator using CuKα radiation. All the samples were scanned at 1°/min in the 2° to 60° 2θ range. Xpert HighScore plus software was used for diffraction patterns and crystal identification.
2.2.2. Textural properties
N2 adsorption-desorption isotherms were obtained at T ≈ 77 K between 0.1 mmHg and 0.99 mmHg using a MICROMERITICS ASAP 2020 surface analyzer. All the samples were degassed for 4 h at 200°C in a vacuum. The BET method and Barrett Joyner-Halenda (BJH) model [17] were used to calculate the specific surface areas and pore sizes, respectively.
2.2.3. Thermal analysis
Thermal characteristics were obtained from thermogravimetric experiments (thermogravimetric analysis and its derivate curves (TGA and DTG)) that were performed using METTLER TOLEDO TGA-SDTA 851 equipment. These tests were performed from room temperature to 1000°C at a 10°C/min heating rate. The sample weight used in all the experiments was approximately 15 mg, and the carrier gas was N2 (99.99%) at 50 ml/min.
3. Results and discussion
3.1 Clay identification
3.1.1 X- ray Analysis
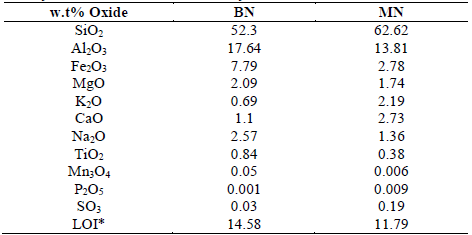
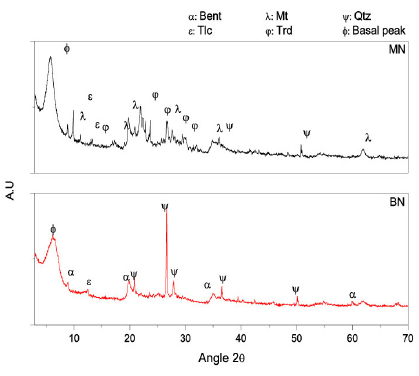
The results of X-ray fluorescence and diffraction of the clays are shown in Table 2 and Fig. 1, respectively. Both the samples have a high content of SiO2 and Al2O3. Clear differences in the composition between the samples were recognized. Organic matter (LOI) contents that were approximately 15% and 12% were found in the BN and MN clays, respectively. The concentrations of SiO2, K2O, CaO, and SO3 of the BN sample were lower than those of the MN sample. Moreover, a higher content of Al2O3 and Na2O in the BN sample (Table 2) suggested sodic bentonite clay, as has been reported by Gong et al. and Boylu et al. [18, 19].
Source: The Authors.
Figure 1 XRD patterns of the commercial clays (Conventions: ϕ: basal peak; ε: talc; α: bentonite; λ: Montmorillonite; ψ: Quartz; φ: tridymite).
X-ray diffraction showed remarkable structural differences between the clays. As expected, the BN samples exhibited typical diffraction peaks of bentonite (Bent) simultaneously with peaks of quartz (Qtz) and talc (Tlc); however, the MN samples revealed typical montmorillonite peaks (Mt), talc (Tlc), tridymite (Trd), and quartz (Qtz).
3.1.2. Thermal analysis
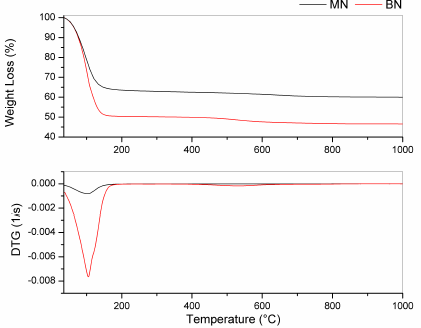
The thermal behavior of both the clays is presented in Fig. 2. A common initial thermal event was observed between room temperature and approximately 200°C, which was associated with water release. However, the offset thermal event was different. The MN offset was approximately 190°C, and the BN offset was 170°C. In addition, weight losses of the clays due to water release were approximately 40% and 50% for MN and BN, respectively. Because of the presence of organic matter, a slight thermal event was observed for the BN samples between 400°C and 600°C.
3.1.3. Textural properties
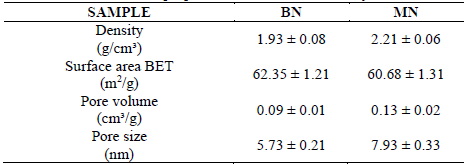
The densities and textural properties of the commercial clays are presented in Table 3. The surface areas were close to 62 m2/g and 61 m2/g for BN and MN respectively. They are low surface areas for catalytic applications; however, the potential of pillarization methods to increase the surface areas has been reported [20]. Regardless, the values of the pore features of the MN samples were higher than those of the BN samples. Moreover, X-ray diffraction revealed that the diffraction patterns exhibited by the MN samples were more than those of the BN samples. This implied that that the structure of the MN sample was more complex than that of the BN sample because of the presence of a diverse phase that could produce adequate disorder to increase the pore size. [21]
3.2. Clay mixtures
3.2.1. X-ray analysis
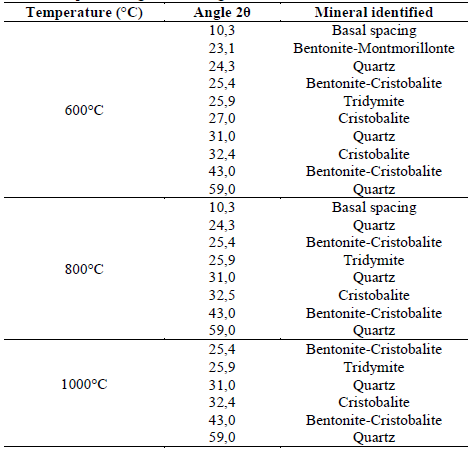
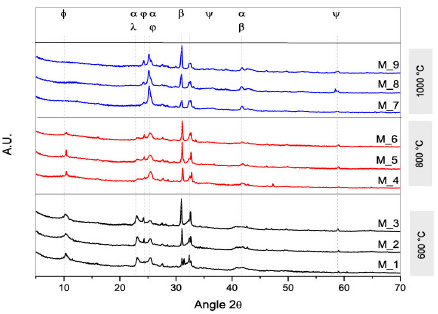
The X-ray diffraction patterns for the mixture of BN/MN clays treated at different temperatures are shown in Fig. 3.
Source: The Authors.
Figure 3 The XRD patterns of the BN and MN mixtures (Conventions: ϕ: basal peak; ε: talc; α: bentonite; β: cristobalitye; λ: Montmorillonite; ψ: Quartz; φ: tridymite).
Structural changes are observed in the samples as a result of the thermal treatment. As the temperature increased, i) the basal spacing intensity at 2θ ≈ 10º was reduced and at 1000ºC, this peak disappeared. ii) The peak intensities of the quartz and bentonite crystals minerals decreased because of the loss of carbonates and alkaline oxides that were present in the original clay. The results agree with the structural changes reported by Bayram et al., 2010 [16], especially for the collapse of the 2:1 (TOT) layer in smectite clays and the conversion of amorphous in crystalline structures at temperatures higher than 1000ºC [22]. Samples M_3, M_6, M_9, had higher intensities in the quartz peak because the concentration of BN (bentonite) was higher in the mixture (75 w.t%).
A summary of the identified diffraction peaks of the mixture of clays have been listed in Table 4 based on the main mineral patterns reported by the International Centre for Diffraction Data for Bentonite and Montmorillonite clays. The ICDD codes of Bentonite, Quartz, and Talc are 00-003-0019, 01-079-1910, and 01-070-2151, respectively, and the ICDD codes of Montmorillonite, Quartz, Talc, and Tridymite are 00-003-0010, 01-079-1906, 01-074-1036, and 00-042-1401, respectively.
3.2.2. Thermal analysis
The TGA/DTFG results of the mixed clays evaluated at different temperatures are presented in Figs. 4-6.
All the samples were treated at 600°C and presented a final weight loss that was approximately 3%. Three thermal events were observed. First, a weight loss below 200°C was observed that was linked with water and gaseous species release, as has been reported by Hedley et al. [23]. Second, a thermal event in the range from 350°C to 550°C that was observed that was due to the dehydroxylation of the clay compounds [16,24]. Third, a thermal event between 580°C and 750°C was observed that was related to a secondary dehydroxylation of the clays. The dehydroxylation destroys the layer structure of the trioctahedral compounds [25].
For mixtures of clays treated at 800°C, a weight gain was observed in the range from the initial (room) temperature to 400°C and an accelerated weight loss was observed at approximately 600°C (Fig. 5). The mixtures with low BN content (M_4) showed more sorption capacity, i.e., approximately 2% wt., than other mixtures studied at this temperature.
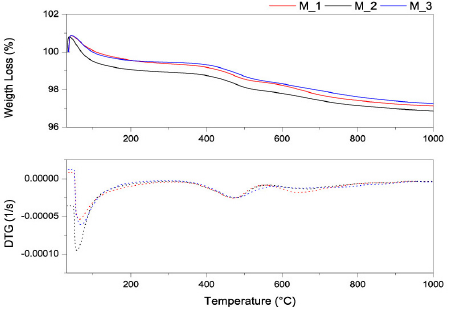
Source: The Authors.
Figure 4 TGA/DTG analysis of the mixtures at different thermal treatments at 600°C.
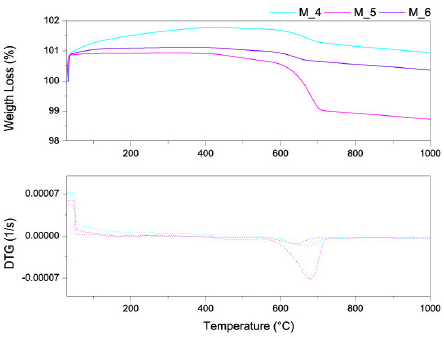
Source: The Authors.
Figure 5 TGA/DTG analysis of mixtures at different thermal treatments at 800°C.
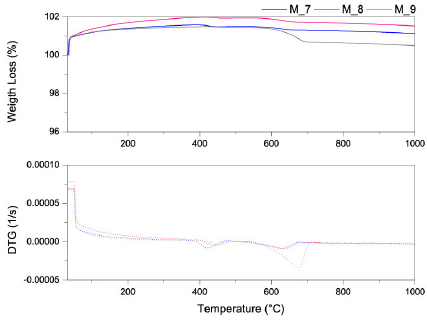
Source: The Authors.
Fig. 6 TGA/DTG analysis of the mixtures at different thermal treatments at 1000°C.
The weight gain was due to the sample sorption of the purge gas (nitrogen). The increase in the sorption capacity was due to surface activation sites from the release of organic substances decomposition and CO2 dehydroxylation, which were both produced by the thermal treatments [26-28]. The observed weight gain was similar to those observed for other natural minerals such as natural zeolites and kaolins [15]. Once the nitrogen sorption capacity was completed, the mass loss of the mixture compounds stabilized.
The mixtures of clays treated at 1000°C show a weight gain between the initial (room) temperature and 450°C (Fig. 6). After this temperature, slight weight loss occurred below 600°C where the gain matter during the testing was lost. Mixture M_8 (50%-50%) had a higher sorption capacity than the others at 1000°C. The weight gain was due to sample sorption of the purge gas (nitrogen) and some explanation exists about the surface activation discussed for the mixtures treated at 800°C.
3.2.3. Textural properties
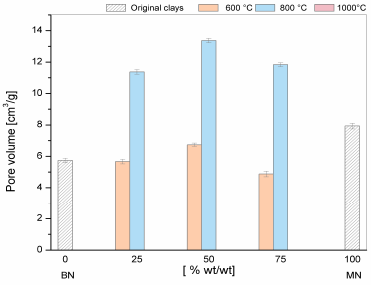
The results of the textural features of the mixtures are presented in Figs. 7-9. For the purpose of comparison, the values of the BN and MN samples are shown at the left and right sides of the graphs. As the temperature of the treatment increased up to 800°C, a) the decrease in the surface area and pore volume was more severe and b) pore size increased. At 100ºC, a clear structure collapse was observed because the porosity was destroyed. These results consistent with the results reported earlier for mineral clays such as kaolin, bentonites, and montmorillonites [13].
The textural results agree well with the DTA/TG data. The surface area and pore volume showed a gradual decrease at 600°C that was consistent with the decrease in pore volume and increase in pore size as a result of the thermal treatment that affected the morphology, layer separation and pore distribution. At 800°C, this loss of surface area is evidently higher for M_5 (50% BN and 50% MN). In this case, the TGA/DTG analysis showed that the maximum rate of dehydroxylation occurred at 700°C. This opened new pores during the dihydroxylation, thereby increasing the pore size compared with the other samples. In turn, the rapid decrease in the surface area and pore volume at the temperatures higher than 700°C was due to the collapse of the 2:1 (TOT) layer structure, as has been previously observed by Bayram [13].
To summarize, thermal treatment of the mixed clays is not a good option to increase their surface areas. However, the porosity of the samples can be improved after they are mixed according to the BN and MN patterns. This is a good option to use these materials as raw and support materials with different porosity ranges in different applications. It involves mixed materials of clays with a large number of holes and different surface properties that are closely related to applications such as support, catalyst, energy storage, chemical reactions, and optical applications.
Thus, thermal treatments and porous density in the improvement of physical properties, electrical and energy efficiency, and the generation of new materials can open an interesting new field of work with the mixed materials of clays.
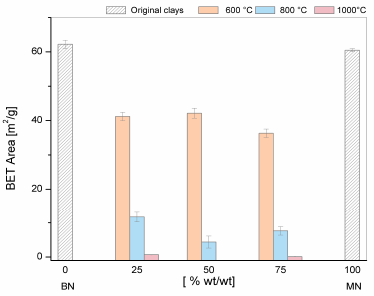
Source: The Authors.
Figure 7 Textural properties of the BET surface areas of the mixtures of clays at different thermal treatments.
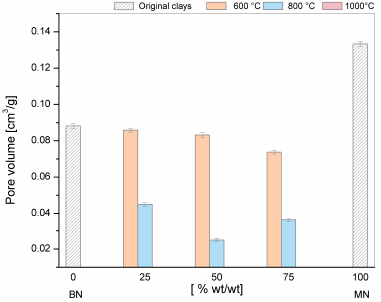
Source: The Authors.
Figure 8 Textural properties of the pore volumes of the mixtures of clays at different thermal treatments.
4. Conclusion
The physical mixtures of two Colombian clays were identified to be bentonite and montmorillonite types using X-ray techniques.. Mixtures of these clays showed physical changes because of applied thermal treatments. Microstructural changes were observed for all the mixtures as a result of the temperature increase. The basal spacing reduced until it disappeared at 1000°C and the peaks of the other crystal minerals decreased because of the loss of carbonates and alkaline oxides in the clays. Considerable reductions in the surface area and pore volume were observed as the temperature increased. The thermal treatment in the range from 800°C to 1000°C led to a loss of surface areas of the all mixtures of clays that were evaluated. All the mixtures that were thermally treated at 800°C and 1000°C showed nitrogen sorption during the thermal analysis that could be associated with the increase in the pore size, which was also observed.