1. Introduction
Corrosion resistance at high temperatures is one of the main requirements for boosting boiler improvement because it increases oxidation in the boiler parts. Boilers undergo corrosion by molten salts, and the temperature leads to an increase in the oxygen diffusion through the molten salt film, causing a reaction in the active elements of the substrate and forming an oxide layer, a process that is amplified if molten salts are present in the corrosive environment. The behavior of carbon steel is not appropriate against corrosion by molten salts because these create porous oxide layers, which are easily removed and accelerate the corrosion process. These oxide layers also reduce heat transfer, drastically affecting the boiler’s thermal efficiency. Hence, the coating of these materials is pivotal for increasing their corrosion resistance and their life span under these environments. In addition, these steel protective coatings will support steel works at 400 °C and 600 °C in thermal plants, thus increasing the thermal efficiency of the power generation process and lowering environmental pollution by pollutant gases such as CO2, SO2, and NOx, as it would also reduce fossil fuel consumption [1-4].
ASTM A53 grade B steel is usually used as tubes, re-heater and over-heater in boilers, which use fossil fuel. This kind of fuel in Colombia content high percent of Vanadium, Sulfur and Sodium [5,6]; hence when they work at higher temperatures than 600 °C, they react with oxygen and become to convert into V2O5 and Na2SO4. The aforementioned information is the main reason for the selection of oxides in this research [6,7].
Arc thermal spray is a metallurgical process by which layers of the same material or other materials are added to a metal. The resulting combination may have better physical, mechanical, and chemical properties or lower costs than the uniform material. This process has advanced through the development of new alloys and processes, which are also highly endorsed in the industry, both in part manufacture and maintenance, thus expanding their application fields. Aluminum is frequently used for protective coatings because it forms an Al2O3 layer, which exhibits remarkable adherence to the substrate and excellent behavior against corrosion. In addition, as low alloy carbon steels possess low chromium content and other alloy elements that may contribute to improving their performance at high temperatures, they require a surface coating to improve their performance and for smooth operation [1,2,9-11].
Because of its low cost and high deposition rate, the arc thermal spray process is widely used to apply coatings to metals and alloys for protection against corrosion and wear [9,11]. However, the main problems presented by coating deposition by thermal spray are the porosity generated and presence of micro and macro cracks generated by the impact speed of the deposition particles when they reach the substrate and by the high cooling rate of the deposit as it reaches the substrate. Nevertheless, if all these variables are adequately controlled, it is very feasible to obtain low porosity coatings by arc thermal spray [12-18].
2. Experimental procedure
In this study, several 15 × 10 × 5 mm ASTM A53 grade B steel test cylinders, which are typically used in boilers, were manufactured. The chemical composition of the steel is given in Table 1. These test cylinders were subjected to a sanding process with emery paper No. 80, 200, 400, and 600 to produce homogeneous surfaces for corrosion tests and a good roughness profile, and thus secure proper adhesion of the coating applied by arc thermal spray. An Al coating was deposited on half of the test samples by electric arc thermal spray. This process was performed at a pressure of 55 psi and at a distance of 20-25 cm; the wire used was aluminum (99.5% pure) with a diameter of 2 mm and deposition flow rate of 2.7 kg/h at 100 A. After the deposition of the aluminum coating, test samples were exposed to heat treatment at 700 °C for 2 h to diffuse the Al throughout the substrate. Finally, the remaining samples were subjected to salt corrosion tests in the delivery state. After preparing all study samples, their weights were recorded to obtain initial comparative measurements.
Table 1 ASTM A53 Grade B Steel chemical composition

Source: Adapted from Mohammad Najafi, PhD. Pipeline infrastructure Renewal and Asset Management. 2016
3. Results and analysis
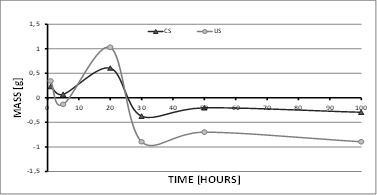
After measuring the test samples extracted during 100 h of the study at 500°C, the coated samples (CS) exhibited gradual mass increase during the first 20 h, but then showed a decrease (mass loss) for the following 10 h before increasing slightly once again until reaching stability around −0.25 g. This behavior is due to the generation of surface oxides and their subsequent detachment, thus leading to the generation of substrate oxides [6,7,18,21]. This phenomenon can be observed between 20 and 100 h (see Fig. 2). On the other hand, the uncoated samples (US) experienced greater mass gain owing to the high generation speed of surface oxides, which easily detached after 20 h, suddenly transitioning from a mass gain of 1 g to a mass loss of approximately 2 g.
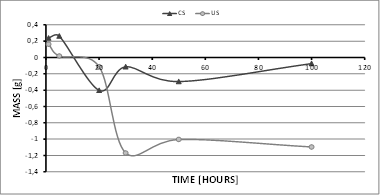
For the tests at 600 °C (Fig. 3), the behavior of the test samples was very similar to that of samples in the tests at 500 °C, with the major difference that the mass loss was greater and occurred in a shorter time (10 h), thus reporting a mass decrease of approximately 1.2 g for US. Likewise, the CS also experienced a mass decrease, reaching values close to those reported at 500 °C. Similarly, the CS managed to stabilize their mass by 100 h, which is a behavior related to the generation of surface oxides [19-26]. In both temperature tests, CS showed a clear mass increase after 20 h of exposure, caused by the generation of oxides, which (as indicated by the SEM images) are not necessarily aluminum oxides (coating), as coating loss is clearly visible after 30 h.
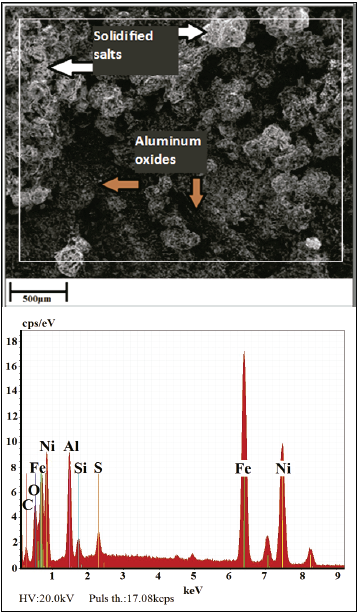
A surface analysis via SEM/EDS of the CS after 20 h at 500 °C is shown in Fig. 4, wherein remains of the solidified salts may be observed, as well as the presence of Al protective oxides, which prevent contact between the salts and substrate until the latter’s oxidation time. The aforementioned is showed in EDS spectra, Fig. 5.
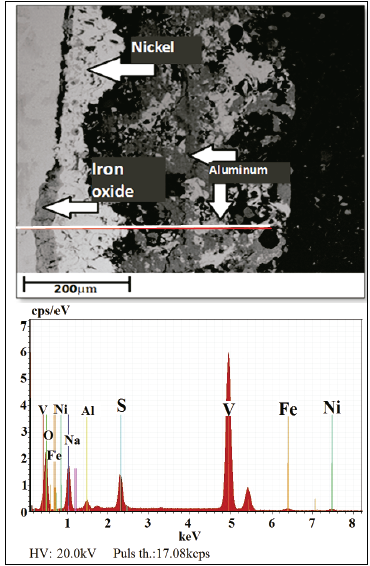
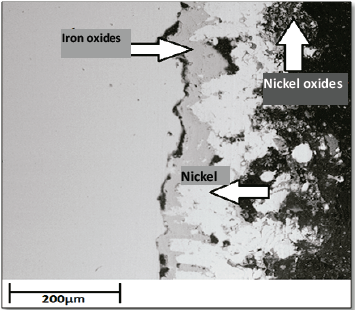
Fig. 5 displays the cross section of a coated sample at a later time (30 h) for the same temperature (500 °C). Therein, the high porosity gained by the coating as well as the detachment of the aluminum layer and its oxides, thus leaving the initially deposited bond layer (nickel) exposed, may be observed. This information is in accordance with results of Villada, LeSung and Yang et al [5-7]. Likewise, the generation of iron oxides on the substrate and the detachment of the nickel layer from the steel are visible in this figure. These findings corroborate the behavior shown in Figs. 2 and 3, for which there are drastic mass gains and losses. Same phenomena was observed by Haiyan and Zhenhua [27-29].
Fig. 6 depicts the coated sample cross section after 50 h at 500 °C, in which the Al layer and its oxides have completely disappeared, leaving only the nickel layer. However, this nickel layer is not sufficient for insulating the substrate from the corrosive environment, since the generation of an iron oxide layer between the nickel and substrate is clearly seen.
The presence of nickel oxides demonstrates the protection provided by the nickel layer against corrosion, since although the generation of iron oxides layers was facilitated by its high porosity levels, nickel continues to exhibit an affinity for oxygen, thus generating oxide layers that protect the substrate until they are totally detached [30-33].
The porosities presented both in the nickel and iron oxide layers will lead to their future detachment, which is consistent with the sudden mass loss observed in the study samples at both temperatures.
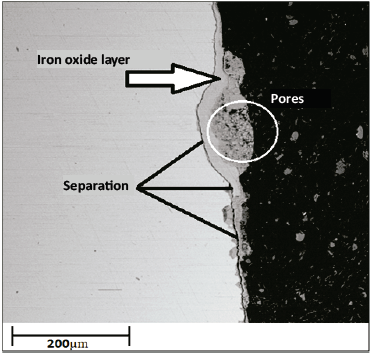
Finally, as expected, at a temperature of 500 °C for 100 h, the coating disappeared completely, leaving the substrate completely exposed to the corrosive environment (see Fig. 7). Therefore, only iron oxides will now be formed. However, these oxides exhibit poor adhesion owing to their high porosities, and separations are observed in the layer-substrate border, which means that their future detachment from the base material is imminent, represented in the notorious mass loss observed in Fig. 2 and Fig. 3.
4. Conclusions
The aluminum coatings deposited by arc thermal spray on ASTM A53 grade B steel showed good protective behavior against corrosion from molten salts [18-20] (Sodium Persulfate+Vanadium Pentoxide) at 500 °C until 30 h because the aluminum layer forms oxides that prevent the substrate from coming into contact with molten salts, thus increasing their life span.
The high porosities exhibited by the aluminum coating deposited by electric arc thermal spray, in addition to the aggressive corrosive environment created at 500 °C and molten salts (Sodium Persulfate+Vanadium Pentoxide), led to the partial detachment of the aluminum protector coating [21-25] after 30 h, with total detachment at 50 h. However, at 600 °C, these phenomena occurred before partial detachment at 20 h and total detachment at 30 h.
The nickel-bonding layer prevented efficient aluminum diffusion throughout the substrate, thus failing to reach the initial purpose of increasing the corrosion resistance of substrate against salts.