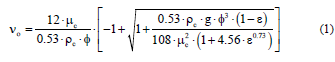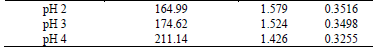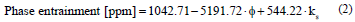1. Introduction
The hydrometallurgical techniques of leaching, solvent extraction, and electrowinning are widely used at the industrial level for recovering metals like copper, cobalt, zinc, or nickel from mining deposits or secondary sources. Solvent extraction (SX) is a technology that has been found to be very versatile for purifying and/or concentrating species from leaching solutions, as well as a technique for treating effluents, in analytical chemistry, and in processes associated with new technologies like compound encapsulation, liquid membranes, and synthesis of nanoparticles [1-3]. Also, the solvent extraction process has been studied in the recycling of Li batteries, recovering Cu and Co from the leaching solutions [4].
Solvent extraction involves a mixture of immiscible phases to promote mass transfer and subsequent phase separation. The phase separation process is a complex phenomenon involving a series of variables factors, such as physical properties, geometric considerations of the reactors and hydrodynamic conditions [5].
The extracting agent, also called organic, is the basic component in solvent extraction processes, and in general it is the highest cost element in an industrial plant, and the reuse and loss minimization of the reagent in question are fundamental. The loss of the organic phase in an SX plant has mainly three causes: organic phase degradation, crud formation, and phase trapping [6-9]. In a commercial SX circuit the continuous replacement of the organic phase is normal due to the losses which are produced along the different stages of the process, even when taking operational measures such as the installation of coalescers, low dispersion stirrers, post-decanters, lipophilic belts in ponds and tanks, among others, [10].
Phase trapping or entrainment is the transport of drops dispersed in a greater volume of the other phase (immiscible) because the drops are not able to coalesce in each time (rupture time). Therefore, trapping of organic in aqueous (O in A) is when the dispersed drops are carried by the aqueous stream, and the opposite case will be called aqueous trapping in organic (A in O) [11]. O in A or A in O entrainment occurs as a result of an imperfect separation of the phases, either for an insufficient residence time in the settler, excessive viscosity of the continuous phase, or due to very small drops that are generated during the mixing and do not have sufficient speed to move through the continuous phase. There are a series of operational factors that can interfere with the trappings, such as, for example, phase inversion, sudden changes of temperature and/or flow of the solutions, degradation of the organic phase, circuit configuration, excessive stirring, and equipment design, among others [10,12,13].
Phase entrainment is an inevitable problem in commercial SX plants, although it is possible to control the problem with certain operational measures, achieving levels between 10 and 100 ppm entrainments. The common problems associated with phase entrainments are impurity transfer, solvent loss, increased generation of dregs, low mixing efficiency, among others [14,15].
Phase separation is a critical process in some hydrometallurgical operations, especially those that need to minimize impurity transference. In the case of copper electrowinning, the rich electrolyte must contain a minimal amount of impurities, for which very selective extractants have been developed. Despite the high efficiency of the solvent extraction process, a certain amount of unwanted impurities (chloride, iron and manganese) are transferred to the rich electrolyte due to phase entrainment. In the case of the chloride ion, when it gets to the EW stage, due to the pH and potential conditions, chlorine gas is generated, affecting the copper cathode with corrosion and contaminating the work atmosphere; manganese, on the other hand, is incorporated in the system as Mn2+ from the leaching of the minerals present in the ore, then in the EW it is oxidized to the Mn4+ or Mn7+ state, and therefore it turns out to be extremely harmful, because it forms precipitates (MnO2) or degradation of the extractant reagent. If the transfer of impurities to the electrowinning stage is not controlled properly, serious operational problems can be generated [14, 16-19], for example:
Increased costs from the loss of solvents.
Oxidation of harmful species like manganese, iron, or chlorine gas in the electrolytes.
Contamination of the leaching heaps by being sprayed with solutions that contain drops of entrained organic, affecting mainly the bioleaching process, where the bacteria are sensitive to the organic compounds.
Decreased chemical quality of the electrodeposited cathode due to contamination with impurities.
Decreased physical quality of the cathodes as a result of the occlusion of impurities and organic spots (burns).
Corrosion of permanent cathodes.
Increased anodic corrosion.
Local contamination in the electrowinning tank house with chlorine gas produced from the evolution of chloride ions to chlorine gas.
Increased costs due to the need of auxiliary equipment (post-decanters, coalescers, filters, etc.).
Because of the problems caused by the phase entrainments described above, commercial solvent extraction plants have implemented a series of mitigating measures such as, for example: low dispersion agitators, reverse flow decanters, organic washing stages, columnar flotation cells, physical barriers inside the gravitational decanter (picket fences), filters, and post-decanters, among others [10,20,21].
The objectives of this study are to determine the trapping of the organic in the aqueous phase when contacting both phases discontinuously, keeping as study variables agitation speed, contact time, pH, and Cu2+ concentration in the aqueous, and extractant percentage in the organic phase.
2. Materials and methods
2.1. Reagents
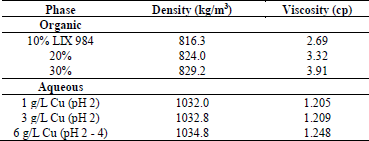
The reagents employed in the preparation of the organic phase are: Shellsol 2046AR as diluent, supplied by Shell Chemicals; and LIX 984N as extractant, supplied by BASF. Both the diluent as well as the extractants are of industrial quality.
To prepare the aqueous phase, copper sulfate pentahydrate (CuSO4*5H20), anhydrous sodium sulfate (Na2SO4), and sulfuric acid (H2SO4) were used, all these reagents of analytical quality. Distilled water was used to dissolve and prepare the electrolytes. The initial properties of the phases used are shown in Table 1. Analytical grade cyclohexane (C6H12) was used for the analysis of the trapping of O in A.
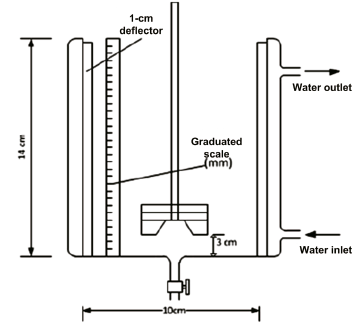
The experiments were carried out in a cylindrical glass reactor temperature-controlled with water, with a total capacity of 1 liter (800 mL effective volume), with an internal diameter of 10 cm and a height of 14 cm, with a flat bottom, equipped with four 1.0-cm wide 316L stainless steel deflectors. The stirrer’s propeller drive, made of 316L stainless steel, has six blades at its base, spaced uniformly on a circular disc and two blades on the surface of the disc, mounted at the center of the mixer-settler at a distance of 3 cm from the bottom of the reactor. The schematic of the reactor and the stirrer is shown in Figure 1.
2.2. Equipment
For the quantification of the organic trapping the following materials were employed: 25- mL cylindrical separatory funnels and Bell model TSB-108 reciprocating shaker with a working frequency of 400 rpm and 10-cm run, provided with holding clamps for tubes/funnels. The phase entrainment analyses were made by UV-VIS spectrophotometry on a Rayleigh model UV-1601 spectrophotometer with a measuring range of 190-1100 nm. The samples were placed in quartz cuvettes, using cyclohexane as blank.
2.3. Experimental procedure
First, the aqueous and the organic phases were mixed for a given time, then the stirring was stopped, the visual separation of the phases was awaited (a time called primary rupture), and once achieved, an organic sample was taken to make the calibration curve (achieving chemical equilibrium), and a 10-mL sample of the aqueous is taken. The aqueous sample is placed in a separatory funnel with the same volume of cyclohexane, then it is stirred vigorously for 5 minutes, allowing the cyclohexane to capture all the organic drops trapped in the aqueous. After the shaking time the cyclohexane is removed and its UV-VIS absorbance is measured at 223 nm, correlating the measured value with the calibration curve.
The average size of the drops was estimated from the sedimentation speed of the dispersed drops, using the follow mathematical expression [22].
The phase separation speed was determined experimentally by recording the phase separation phenomenon with a Sony model Flea 2.0 scientific video camera with a monochromatic 2/3” CCD sensor and 12.5 mm Fujinon optics. Then the speed at which the advancing front presents a linear behavior (initial stretch) was determined exactly, where υo corresponds to the initial phase separation, ϕ is the average diameter of the drops, ρ and μ corresponds to the density and viscosity (the subindex c refers to the continuous phase), and ε is the de dispersed phase fraction.
3. Results and discussion
To quantify the O in A entrainment, the variables: stirring rate, shaking time, pH, extractant concentration in the organic phase, and copper concentration in solution were studied. The experiments were performed at 20 ºC, aqueous continuity, and phase relation (O in A) 1:1. In each of the tests, samples were taken for UV-VIS analysis, and the phase separation speed was recorded to estimate the average diameter of the drops.
3.1. Effect of stirring rate
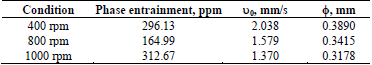
To evaluate the O in A entrainment as a function of the stirring rate, three values were considered: 400, 800, and 1000 rpm, the mixing time was set at 3 min, and the electrolyte had an initial pH of 2. Table 2 shows the results of evaluating the stirring rate, which showed a parabolic trend in terms of phase entraining, where at 800 rpm a minimum value of 165 ppm was found, with the explanation lying in the effect of the phase separation rate and the generation of a fraction of small sizes. When the mixture was stirred at 400 rpm, the phase separation occurred very rapidly (2.038 mm/s), the advancing front moved at greater speed than many dispersed drops which cannot advance at a sufficient rate, finally getting trapped in the electrolyte. On the other hand, stirring at 1000 rpm implies the generation of a large fraction of very small drops (average drop diameter of 0.3178 mm) which do not reach sufficient speed no and remain entrained in the aqueous phase, reaching an entrainment value of 312.37 ppm.
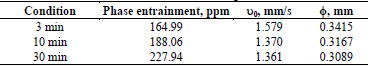
3.2. Effect of stirring time
The effect of stirring time was studied considering three levels: 3, 10, and 30 minutes of stirring; at 800 rpm the electrolyte has an initial pH of 2. The experimental results are shown in Table 3, where a clear trend is seen as stirring time increases, the entrainments of O in A also show it, reaching a maximum of 227.94 ppm. The results are attributed to the increase of the fraction of small drops, which are generated as the result of the rupture caused by stirring, so the average drop diameter decreases from 0.3415 mm to 0.3089 mm, implying that excessive mixing increases the probability of mechanical rupture of the drops. It should be noted that the minimum mixing time of the phases recommended by the manufacturers is 3 min, and below this time the extraction efficiency decreases dramatically.
3.3. Effect of initial pH
To evaluate the organic phase entrainment as a function of the initial pH of the aqueous solution, three pH values were considered: 2, 3, and 4, and the phases were mixed at 800 rpm for 3 minutes. The results are shown in Table 4, showing an increase of the O in A entrainment as the initial pH of the aqueous phase increases. The explanation of this behavior is that it is due to the displacement of the chemical equilibrium (the copper extraction changes from 69.16% to 71.26%), since the properties of the aqueous phase do not change in this range. The displacement of the equilibrium causes an increase of the sedimentation front rate from 1.579 mm/s (pH 2) to 1.744 (pH 4), causing a larger amount of organic drops to avoid coalescing and remaining entrained in the aqueous phase because they will not reach sufficient speed to reach the sedimentation front.
3.4. Effect of the percentage of extracting agent
To evaluate the effect of extractant percentage in the organic phase entrainment, three extractant concentration levels (10, 20, and 30% v/v) were studied; the phases were mixed at 800 rpm for 3 min, with the electrolyte at an initial pH of 2. The results are summarized in Table 5.
Table 5 Quantification of phase entrainment at different extractant concentrations.
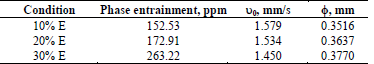
Source: The Authors
The results show that as the extractant concentration in the organic phase increases, so do the phase entrainments directly; it is presumed that this effect is due to the influence of the extracting agent on the physical properties, since as the concentration of LIX 984 increases, so do the viscosity and the density of the organic phase, implying that the dispersed drops move more slowly, and joining their corresponding phase becomes more difficult.
3.5. Effect of the copper concentration in the aqueous phase
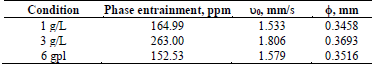
The effect of copper concentration in the aqueous electrolyte at three initial levels (1, 3, and 6 g/L) was studied; the phases were mixed at 800 rpm for 3 min, at an initial electrolyte pH of 2. The results are summarized in Table 6.
Table 6 Quantification of phase entrainment at different copper concentrations in the aqueous electrolyte.
Source: The Authors
The results show that as the copper concentration in the aqueous phase increases, phase entrainments would also increase, peaking at a copper concentration of 3 g/L. The explanation lies in that the aqueous phase changes its physical properties, since as the copper concentration increases so do its viscosity and density, hindering the motion of the dispersed organic drops that are generated and must be displaced through the continuous phase (aqueous). Furthermore, the properties of the organic phase strongly depend on the transfer of copper from the aqueous.
3.6. Multivariable statistical model
A statistical analysis of all the variables that were included in this study was made with the purpose of determining which are the most influential in the phase entrainment phenomenon. A multivariable statistical analysis was made, from which it was determined that the phase entrainments depend significantly on parameter ks and on the average diameter of the drops (φ), discarding the influence of other parameters like the final phase separation time, the sedimentation time, or the packing factor, to name a few. This result is completely logical, since the sedimentation speed and the Sauter diameter represent directly the effect of the physical properties of the phases and the system’s hydrodynamic conditions.
Once the most significant variables have been established and the less significant ones have been discarded, a multivariable regression was carried out, delivering a statistical model that predicts phase entrainments, as shown below.
The model obtained indicates the existence of a statistically significant relation between the studied variables and the O in A entrainment level, with a confidence level of 95.0%. Clearly, the statistical model presents certain limitations, the most important of which is that it is only applicable to the studied system and under the studied conditions, but yet it is a tool that can be used as an approximation to the study of O in A phase entrainment.
4. Conclusions
As the experiments have shown, it can be concluded that phase entrainment is a phenomenon strongly influenced by the physical properties of the phases, in addition to a component associated with the hydrodynamics of the system.
Analyzing the results obtained for the stirring rate, it was determined that the phase entrainments do not increase as a function of the speed, as it was expected. At low rpm, the entrainment is high, due to the fact that the advance front moves quickly; this is because the system cannot break the drops too much. In contrast, at high rpm, the system generates very small drops, which cannot move at a sufficient speed, causing an increase in the organic entrainment. The stirring time influences the probability of mechanical breakage of drops, since the longer the stirring time, the greater the probability that a drop will be reduced in size by mechanical action.
Mass transfer is very relevant in the phase entrainment phenomenon, this is due to the change in the physical properties of the phases. The chemical equilibrium of the extraction (or stripping) reaction induces changes in the physical properties of the phases, the greater the mass transfer, the greater the increase in density and viscosity of the organic phase, implying a greater entrainment level.
Finally, it was verified that there is an important effect between the initial physical properties of the phases and the phase entrainment. As the density and initial viscosity of the phases increase, the phase entrainment increases significantly. In the case of the increase in the extractant content in the organic phase, the gain in entrainment is attributed to the fact that the drops generated are denser and heavier, so their movement is slower. In the case of increasing the concentration of copper in the aqueous phase, the boost in entrainment is attributed to the fact that the electrolyte becomes more viscous, so that the organic droplets move with greater difficulty through the continuous phase.