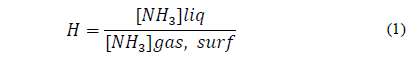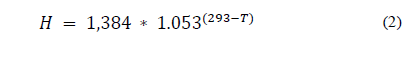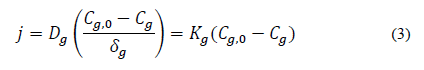1. Introduction
Brazil is currently the second-largest producer of chicken meat worldwide, reaching 13.05 million tons in 2017 and being surpassed only by the United States, which produced approximately 18.5 million tons [1]. According to previous research [1], 66.9% of Brazilian chicken meat production was destined for the domestic market, reflecting a per capita consumption of 42 kg habitant-1 year-1 in 2017.
The production of broiler chickens generates, directly or indirectly, a large amount of pollutants at all stages in the production chain, from the cultivation of grain for animal feed to the generation of residues, such as manure, litter, dead animals, and slaughterhouse effluents [2]. Such residues contain significant amounts of nutrients, such as nitrogen, phosphorus, potassium, copper, and zinc, as well as a high bacterial count [3] that affects the degradation of these elements, generating environmentally harmful compounds. In the broiler productive cycle, ammonia is the main gas generated, being present at higher concentrations compared to that of other gases. Therefore, it is of concern among producers and researchers. In addition to ammonia being a toxic gas to humans and animals when at high concentrations, it also causes negative impacts on the environment [4] by acting as a greenhouse gas [5].
The main source of ammonia production in broiler houses is the nitrogen found in urea, uric acid, and protein. These proteins are present in the undigested portion of excreta and are derived from amino acid-rich diets that ensure that the nutritional needs of the birds are met [6,7]. In general, many factors influence the concentration of ammonia inside poultry houses, such as housing density, nutrition, waste management, ventilation, temperature, and relative humidity of the air and near the floor [8,9]. Ventilation is one of the most important actions in the control of the ammonia concentration in these facilities. In addition to reducing climatic effects, ventilation is required to remove excess moisture from the air and litter. Consequently, it allows the air to be renewed by removing the toxic gases generated in the facility [10].
The poultry farming environment is a determining factor in the success of the production, given the climatic conditions and air quality. Therefore, it is essential to study the origin and trajectory of nitrogen during broiler rearing. By understanding the entire production system, it could be possible to develop mitigating measures to minimize ammonia emission and its impacts on the environment. Thus, it would be possible to ensure that productivity levels, animal welfare, and sustainability of activity are maintained.
The objective of this literature review was to address aspects related to the origin, percentages, retention forms, and disposition of nitrogen in broiler houses. Overall, the objective was to determine the stages of ammonia transformation in the microenvironment, from the source material to gas generation.
2. The nitrogen cycle
Nitrogen is one of the most important elements on earth because it is essential in amino acid and protein composition, which act in enzyme formation, tissue building, antibody formation, and hormone production, among other processes. It represents 78% of the volume of the earth's atmosphere. Its name is derived from the Greek “nitrogenium,” and it is also known as “azoto,” which in Greek means "lifeless" because of its inertia [11,12]. However, despite its abundance in the atmosphere, the amount of nitrogen in the earth's crust is relatively low, being approximately 19 ppm [12]. Only 0.02% is available for use by vegetables [13].
Because of its low reactivity, most life is unable to utilize molecular nitrogen (N2). A large amount of energy is required to break its triple bond. Therefore, molecular nitrogen must be converted to a more reactive compound, such as ammonia, ammonium, or nitrate. This conversion is accomplished through the fixation of atmospheric nitrogen, which is the process by which atmospheric nitrogen gas is aggregated with nitrogenous organic compounds and is thereby included in the nitrogen cycle [13].
Nitrification, denitrification, nitric oxide formation, nitrate leaching, and ammonia volatilization (NH3) processes are possible transformation routes for nitrogen molecules [7]. According to one study [14], the nitrogen cycle involves several stages, which, overall, can include:
Fixation: The process by which atmospheric inert nitrogen becomes available to living organisms (plants and animals) by becoming fixed in tissues of other living or dead organisms. Leguminous plants and some bacteria can fix nitrogen directly from the air. Bacteria are responsible for this process, resulting in the reaction of nitrogen and hydrogen to form ammonia.
Ammonification: Part of the ammonia present in the soil is derived from fixation. The other part comes from the process of decomposition of proteins and other nitrogen residues contained in dead organic matter and excreta. Bacteria and fungi present in the medium perform ammonification (or decomposition).
Nitrification: This consists of a series of reactions in which some species of microorganisms oxidize the ammonium ion (NH4+) to nitrite (NO2-) or nitrite to nitrate (NO3-). It is called autotrophic when initiated by ammonia (NH3) and performed by autotrophic organisms.
Denitrification: At this stage, denitrifying bacteria, such as Pseudomonas denitrificans can convert nitrates into molecular nitrogen, which returns to the atmosphere; thus, completing its cycle.
According to [13], modern man has altered the nitrogen cycle by introducing large amounts of reactive nitrogen into the environment, mainly because it is an essential nutrient in agriculture. Livestock breeding, especially in intensive systems, is one of the processes that incorporate high amounts of nitrogen into the environment. Particularly in the poultry industry, birds are primarily fed protein-rich diets with nitrogen content beyond what is necessary to meet their nutritional needs. Animals do not metabolize much of the ingested nitrogen. This is because of their food conversion, which is often inefficient. Thus, nitrogen is excreted and exposed to microbial activity, which generates gases with potentially harmful effects when present at high concentrations in the environment, such as ammonia from the decomposition of poultry manure [15].
3. Nitrogen from broiler diet
[7] stated that broiler performance is directly influenced by the feed quality. Recommendations for feed composition are defined to aid the attainment of certain body mass values according to the age of the bird and to achieve maximum conversion efficiency. For this, it is necessary to offer diets with high energy levels and high crude protein content. As an example, the recommendation of feed formulation, according to the different phases of the broiler rearing cycle, suggested by Cobb-Vantress for Cobb500® broilers, states that according to the age of the birds, different nutritional recommendations are proposed to meet the needs of the animal [7]. Crude protein levels in the diet decrease with increasing age, while the energy level increases. This may be explained by the higher feed conversion rate of birds with increasing age, which implies higher feed intake to maintain the body mass gain of older birds compared to that of younger birds [7].
The utilization of dietary protein begins with protein digestion in the poultry proventriculum and ends in the transport of amino acids and peptides through the basolateral membrane of the small intestine. During passage through the crop, acidogenic bacteria partially denature the proteins. However, protein digestion is only achieved when it reaches the stomach. The stomach of the birds is divided into two parts: gizzard and proventriculum. The presence of food intake in the proventriculum promotes the production of hydrochloric acid and pepsinogen. The gizzard acts as a mechanical organ, without excretion or absorption, but is very important in mixing food intake with water, saliva, hydrochloric acid, and pepsin [16,17].
After absorption, amino acids are transported to the liver, and some are used in the synthesis of liver tissue proteins or blood proteins. Most amino acids cross the liver in the form of free proteins. They are later made available to other body tissues (especially muscle tissue) for protein synthesis [17]. Additionally, another purpose of amino acids is catabolic degradation for the release of energy to be used in biomolecule synthesis [7].
Among the structural functions of proteins, is the action on the composition of the bone matrix, connective tissue, and muscle tissue, among others. All different protein types are polymers of only 20 amino acids joined by peptide bonds [16,18].
Protein is the most expensive nutrient in a feed and a key element for the good performance of animals. Protein utilization depends on the amount, composition, and digestibility of its amino acids. Unlike carbohydrates and lipids, proteins do not have an amino acid reserve mechanism, and all excess amino acids supplied in the diet are catabolized. Metabolism of excess amino acids generates higher energy expenditure for excretion, causing an unnecessary caloric increase, thereby compromising the performance of the animals [16,19]. For a long time, poultry feed formulations were based on the crude protein concept, culminating in diets with high amino acid levels, exceeding the values required by the animals. Thus, once metabolized, the derivatives of these proteins became the precursors for uric acid formation, which, when excreted, is easily converted into ammonia [16].
From the industrial production of amino acids came the concept of an ideal protein, based on which rations were formulated, with a protein level closer to ideal levels. An ideal protein is one that achieves the exact balance of amino acids, without excess or scarcity, to meet the needs for maintenance and good protein utilization, reducing the use of amino acids as a source of energy and minimizing nitrogen excretion [17,20].
4. Nitrogen retention forms in broiler chickens
The performance of birds is directly related to the feed offered to them [7]. The purpose of poultry feed formulation is to adjust the amount of nutrients to that required by each strain according to the desired production level [17,21], to minimize excretion losses. Amino acid deficiency in the early phase prevents maximum protein deposition in tissues. On the contrary, excess nitrogen at the end of the production cycle is excreted as uric acid, generating nutrient waste, and additional caloric expenditure for the excretion process [22].
Nitrogen is a fundamental part of amino acid composition, and amino acids are used for protein synthesis. Except for water, proteins are the most important and abundant molecules in the body, because they perform numerous vital functions, being the main constituents of cell structure. They are used as nucleic acid precursors, coenzymes, hormones, and antibodies, among others. They are the basic elements for the formation of blood cells and are mainly used in the formation of organs and muscle tissue [18].
Of all nitrogen present in broiler feed, the percentage of carcass deposition can be influenced by animal characteristics, environmental conditions, and nutritional factors, as well as the age and gender of the birds [23]. In general, young birds have accelerated growth and during this period there is an intense deposition of muscle protein; thus, there is higher nitrogen retention in the carcass. According to [24], higher nitrogen retention is common in chicks. Because they are growing, they use the nutrient for deposition of proteins in tissues. Similarly, [25] observed higher nitrogen retention in younger broilers, with gradual deposition increase until 28 days of age. After this, nitrogen retention tends to decrease, indicating that growing animals require higher nitrogen levels because of the high nutrient deposition in protein tissues.
Lysine is the most abundant amino acid in broiler muscle, constituting an amino acid of great importance for the construction of skeletal muscle tissue [26]. Protein deposition in animals increases with increasing digestible lysine in the diet [27,28]. [29], while determining the lysine utilization efficiency for the two genders of Ross® broiler chickens, observed a variation from 49.6 (1 to 7 d) to 56.1% (36 to 42 d) in the efficiency for males and a variation in lysine utilization efficiency ranging from 55.0 (1 to 7 d) to 57.4% (36 to 42 d) for females. [22], evaluated different levels of lysine in the broiler diet of both genders of broiler chickens during the entire productive cycle and observed higher protein deposition in carcasses of males in the final phase of production. [30] estimated the presence of approximately 45.9% protein in broiler carcass, whereas [31] observed 51.8%.
5. Nitrogen disposition by broilers
The main means of nitrogen entry into the broiler rearing system is feeding. For a long time, the formulation of poultry feed was based on the concept of crude protein, according to which the amount of protein in the feed was defined by the feed nitrogen content multiplied by 6.25 [32]. As a result, diets had higher amino acid levels than required by the animals and excess nitrogen was eliminated through excreta [33,34]. Currently, it is possible to maintain poultry performance by decreasing protein in the feed formulation based on the concept of ideal protein [32].
According to [18], nitrogen not used for digestion is passed to the liver and converted to uric acid for excretion. According to these authors, the amino acids and proteins ingested by animals are sent to cells and are assigned to two different functions: synthesis of other amino acids and proteins for deposition in various tissues, especially muscle and catabolic degradation for energy release and use of that energy in biomolecule synthesis. Nitrogen found in bird fecal waste is excreted as uric acid (approximately 50%) [32], as well as undigested protein. Microbial degradation of uric acid in excreta is the main source of ammonia formation in a poultry facility. Bacillus pasteuri bacteria, which conduct the decomposition process, require a pH of approximately 8.5 for optimal growth. Additionally, the process involves enzymes, such as uricase and urease. Uricase converts uric acid to allantoin, which is later converted to glyoxylate and urea. Urease, in the presence of water, breaks down urea into ammonia (NH3) and carbon dioxide (CO2), and the formation of NH3 is followed by decomposition of waste under aerobic and anaerobic conditions. Because it has no ionic charge, ammonia in gaseous species is easily released into the atmosphere [35].
In addition to excreta, animal mortality also represents an important source of nitrogen loss in the system and can reach expressive values, especially in high-density rearing systems. Mortality is dependent on factors, such as housing density, thermal conditioning, and bird age, among others [36]. Therefore, the mortality rate should be accounted for in the nitrogen balance calculations in poultry houses [7].
Nitrogen loss in a broiler rearing system can be estimated using the concept of mass balance [7, 37]. In this method, the nitrogen inputs and outputs of the poultry house are quantified and based on the algebraic difference between the amount of nitrogen present in the feed, litter, and poultry, its loss is calculated. However, it has a disadvantage in that this method does not specify losses of N in the form of NH3, N2, or NOx [7].
Nitrogen is easily found as nitrate and ammonia in poultry houses. According to [38], undigested poultry protein is converted to ammoniacal nitrogen through bacterial activity, resulting in ammonia (NH3) and ammonium (NH4 +) formation. Through nitrification, NH4 + is transformed into nitrate, which, because it is water-soluble [39], is the main form of groundwater contamination. Excess nitrogen and other nutrients lead to soil acidification and eutrophication of water bodies [2,40,41].
[42], studied the performance of broilers fed low-protein diets, which were raised at different temperatures and observed that low-protein diets impaired performance and nitrogen retention efficiency of broilers raised under heat stress. However, low-protein diets may be offered to broilers raised in environments with air temperatures between 20 and 25 °C, because no changes in performance or carcass quality were observed, in addition to the minimization of nitrogen excretion. The authors pointed out that, under thermoneutral conditions, animal fed diets formulated with 15% crude protein excreted only 11.3% and 23.8% of the ingested nitrogen when housed in environments with air temperature maintained at 20 °C and 25 °C, respectively. However, poultry subjected to heat stress (32 °C) excreted approximately 63% of the nitrogen from the diet [42].
According to [43,44], waste accumulation time influences N concentration. Fresh excreta have higher N content that, over time, can be mineralized and volatilized in the form of ammonia.
6. Steps involved in the transformation of nitrogen to ammonia
Because most poultry diets have high-protein levels, excess nitrogen, which is unused by the body, is eliminated with the feces and is later degraded, releasing ammonium (NH4 +). This, in turn, is converted to ammonia gas (NH3), which is highly volatile, resulting in nitrogen losses to the atmosphere [38]. Birds excrete nitrogen in solid substances, more precisely in the uric acid form. It is possible to verify, in poultry excrement, a larger and darker lower plate (feces), and a smaller white upper plate, which is uric acid. Thus, bird excreta is composed of feces and “urine,” and therefore, they is richer in nitrogen when compared to swine and/or cattle feces [45].
In production systems where animals are kept in confinement, the air quality inside the facilities becomes a limiting factor in production. High-ammonia concentrations can substantially affect poultry performance [46], and therefore, is a major concern from both an economic and environmental standpoint. According to [47], NH3 volatilization from animal waste is the second-largest source of ammonia emission into the atmosphere (approximately 32 Tg N year-1), only behind that of wet deposition (approximately 46 Tg N year-1).
Ammonia is the product of the microbial decomposition of uric acid excreted by poultry. According to [46], there are numerous biological, physical, and chemical processes involved in ammonia generation and emission. Considering the broiler litter layer as a control volume, these processes, briefly, include ammonia generation, the division between the adsorbed phase and the aqueous phase of ammonium ions, balance between ammonium ions and free ammonia in the aqueous solution, division between the solid/aqueous phase and the ammonia gas phase, and convective mass transfer of ammonia from the surface to the atmosphere [46].
Nitrogen excreted by broilers is mineralized by bacteria, releasing ammonium ions (NH4 +) from the litter. NH4 +, in the presence of moisture and oxygen, results in the formation ammonia, which is a highly volatile substance that is easily released into the air [15]. According to [7], under favorable environmental conditions and excreta deposition on the litter, the bacteriological degradation process begins, resulting in the formation of simpler compounds. This process occurs as long as the raw material is available and the final inorganic substances are volatilized or assimilated by other living things, such as vegetables if they are used as fertilizer. The main process that constitutes the N biogeochemical cycle in a poultry facility is mineralization, which is defined, according to [48], as a complex set of chemical reactions, governed mainly by biological means, where an organic substrate is converted into living biomass and mineral waste.
Ammonium ions in broiler litter are divided into the adsorbed and dissolved phase. Ammonia dissolved in the liquid portion of the litter surface may exist in the ammonium ion form and as free ammonia. This relationship is expressed by the dissociation constant (Kd). According to [49], for the prediction of the equilibrium ammonia gas concentration over manure slurries and litter, [50] determined a disassociation constant of ammoniacal-N, and [51] developed Henry’s Law constant. Henry's constant (Kh) describes the equilibrium between free ammonia in the aqueous and gas phase [45]. According to [52], this constant characterizes the ratio between a population of molecules of a given compound in two phases, determining the relative compatibility of the compound for each medium to the equilibrium between the vapor and solution phase. To [49], to determine the concentration of ammonia in the air, which is in equilibrium with free ammonia in the liquid film on the free surface of water and air, Henry's law is used (Eq. 1). According to these authors, Henry's Law constant is also affected by temperature (Eq. 2). With the convective mass transfer, part of the gaseous ammonia from the broiler litter surface is transferred to the facility atmosphere [45].
Where:
[NH3]liq: concentration of free ammonia nitrogen in liquid (kg/m3); [NH3]gas,surf: concentration of free ammonia in the gas at the liquid film at the water-air free surface (kg/m3); H: Henry’s constant (dimensionless).
The litter ammonia emission process involves the transportation of the aqueous phase or solid phase of ammonia to the gaseous phase in the atmosphere. The poultry litter ammonia volatilization rate is directly linked to NH3 dissolved in the solution that surrounds the litter particles. In contrast, the availability of ammonia in the broiler litter solution is influenced by factors, such as pH, humidity, temperature, and NH4 + concentration. Under these circumstances, NH4 + (which has low volatilization) is converted to NH3 (which is a highly volatile compound) [7].
Ammonia from broiler litter is volatilizable into the air circulating in the environment. Before being emitted into the atmosphere, ammonia is enveloped at equilibrium in the liquid (l) and gaseous (g) phases (Reactions 1-3) [53].
The balance between ammonia and ammonium ions is influenced by temperature (T) and pH. At pH values below 7.0, almost all ammonia is bound to ammonium and is not subject to volatilization. The manure pH value is decisive because it establishes the ammonia concentration in the aqueous or gaseous phase, influencing its release. Ammonia emission begins when pH is close to 7 and increases significantly when pH is greater than 8 [46]. At pH lower than 7, the H+ ions in the litter increase the ammonium: ammonia ratio, which means that more ammonia will be converted into nonvolatile ammonium ions [54].
Air temperature influences the coefficient of convective mass transfer and litter temperature may interfere with Henry's constant, the dissociation constant, and also the diffusion and generation of ammonia in the litter [45]. Elevated temperatures favor ammonia concentrations because it influences the dissociation constant Kd. The volatilization equilibrium of gaseous ammonia follows Henry's Law for dilute systems.
The partial pressure of gaseous ammonia, NH3 (g), is proportional to the concentration of liquid ammonia, NH3 (l). Ammonia volatilization from litter to air is defined as mass flow. This mass flow can be defined as the product of the difference in the partial pressure between the two media and a mass transfer coefficient. A higher partial pressure difference increases flow. Mass transfer coefficients increase as air velocity increases [53].
For the interphase transport system, there are many proposed theories, notably the Two Film Theory by Welty and Boundary Layer Theory by Olesen and Sommer, which gave rise to a general equation for the transfer flow of mass [7,45].
a) Two Film Theory. According to [7], this theory assumes that:
According to [55], the NH3 transfer process is comprised of three steps, namely:
Diffusion of NH3 from the interior of excreta and litter to the surface;
NH3(l) transfer through the surface liquid layer;
Convective transfer of NH3 (g) between a thin air film and then to air.
[56] proposed a process-based model that describes the turnover of organic matter and gas emissions, called the Manure-DNDC. In this model, the authors applied the Two Film Theory to the prediction of ammonia emission from the liquid surface of excreta into the air. [57] used ultrasonication to reduce ammonia levels in livestock waste, thereby improving anaerobic digestion efficiency. To model the mass transfer, [57] used the Two Film Theory, and from this, they estimated the ammonia mass transfer rate.
a) Boundary Layer Theory: As stated by [7], this theory suggests that NH3 volatilization occurs in two stages:
Furthermore, [7] reported that [55] used Fick's Law (Eq. 3) to mathematically represent the ammonia transfer from the liquid surface surrounding the litter particles.
where:
j (kg m-2 s-1): is the NH3 mass flow; Dg: NH3 diffusion coefficient in the air; Cg: NH3 concentration (kg m-3) above the boundary layer; Cg,0: NH3 concentration in the boundary layer underlying the surface of the liquid layer surrounding the manure particles or litter; δg: laminar boundary layer thickness (m); Kg: convective coefficient of NH3 transfer in the air above the boundary layer (m s-1).
According to the report by [58], the boundary layer theory refers to the resistance that limits ammonia transfer from excreta to free airflow. It is most commonly used to estimate the gas phase mass transfer coefficient in controlled environments [58]. They evaluated the effect of different wind tunnel geometry on boundary layers and their effect on ammonia emission. It was observed that as the boundary layer thickness increased with the wind tunnel size, a higher boundary layer thickness increased the resistance of the mass transfer process from the emission surface, corroborating the boundary layer theory.
The main variables that affect NH3 volatilization are pH, relative humidity, and litter temperature. As the availability of poultry excreta in litter increases, so does the microbial population that acts on organic matter mineralization and uric acid transformation in the litter, with consequent increases in its NH4 + content [7]. In the opinion of [45], there are still other factors that present a major influence on the ammonia emission rate to be considered, such as litter material, nitrogen content in poultry feed, animal gender, breeding density, rearing time, age of birds, litter thickness, presence of aerobic and anaerobic microorganisms, urease enzyme, renewal rate, air velocity, and reuse of litter.
Producers must be aware of all these factors to ensure good air quality and, when necessary, take appropriate measures to maintain an appropriate indoor environment for good poultry performance. The breeding environment must provide conditions in which the animal spends the least energy to adapt to the environment, as well as promote better welfare for both poultry and workers.
7. Final considerations
From this review, it is clear that broiler production results, directly or indirectly, in a large amount of pollutants at all stages of the production chain, and ammonia is the main gas produced. Thus, it is important to understand all the processes involved in its generation and how it affects the health and welfare of animals and humans present in these environments.
Furthermore, the nitrogen present in urea, uric acid, and proteins undigested by poultry is the main source of ammonia generation in the production system. Once excreted, it is decomposed and releases, as products, ammonium or ammonia gas. Another major nitrogen exit pathway in the system is mortality. Temperature, humidity, and pH are crucial in ammonia production in poultry houses. In addition to these, many other factors greatly influence the processes of ammonia generation, transformation, and volatilization, such as rearing density, age of the animals, litter thickness, and presence of microorganisms, among others.
The path taken by nitrogen in the broiler production system involves several stages, the most important of which are the synthesis of uric acid and excretion by poultry; mineralization of nitrogen present in excreta; volatilization of ammonia present in the litter.
Therefore, producers need to be able to identify the variables and understand the processes that cause the highest generation and emission of ammonia, so that appropriate management techniques can be adopted to maintain good air quality in the microenvironment of production units.