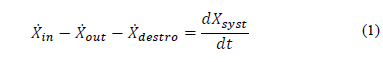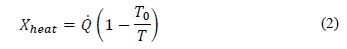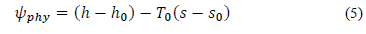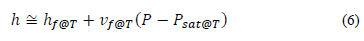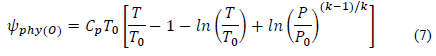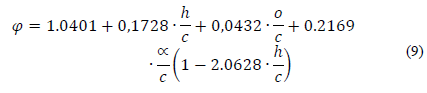1. Introduction
Nowadays, the most important energy sources come from fossil resources [1] and their derivatives, such as oil, gas and primary energies, in order to obtain final energies. However, this practice is not sustainable due to population increase [2,3] and, consequently, to the high production and generation of energy that is required. According to the above, from the year 2000, the increase in ppm of CO2 [4,5] as a result of excess energy consumed unnecessarily, has provided a significant increase in global pollution [6] and if this continues, by 2050, this will have increased the Earth’s temperature to 2°C, at which point, changes are irreversible [7,8]. Consequently, strategies are proposed to ensure that this temperature increase does not exceed 0.5°C, which would be achieved by various means.
One of them comes from reforestation, which involves planting trees, as well as from generating electricity from nuclear energy [9] and renewable energy, thus leaving the possibility of significant change to the efficient use of energy.
For this reason, it is crucial to focus on the increase of renewable energies and their efficiency, for which it is common to use hybrid systems, as well as to study their thermodynamics. An answer to this would be thermoelectric generators (TEG), which can generate a voltage differential and thus obtain electric energy due to a temperature differential [10]. Because of its important applications, authors such as Murat et al. [11] have focused on thermodynamic studies that determine the energetic and exergetic efficiencies of each subsystem composing it. Similarly, Islam et al. [12] examined the operating conditions of a system, as well as its energy and exergetic influence on solar panels, such as in thermoelectric devices and concluded that the system presents a significant improvement by including thermoelectric generation.
On the other hand, hydrogen production using a proton-exchange membrane electrolyzer (PEM) provides a promising way to store and make better use of renewable energy resources. Nowadays, various studies on electrolyzers have focused on improving their energy and exergetic efficiencies to promote their technological progress. A. Kazim [13] performed an exergonomic analysis on a 12900 kW PEM electrolyzer, evaluating its performance under different operating temperatures and pressures; it was found that a 40% improvement in the exergetic cost of hydrogen can be achieved if the electrolyzer operates at low temperatures. However, a 2% decrease in the cost of exergy could be achieved if the operating pressure is increased from 1 to 10 atm. M. Leung et al. [14] developed energy and exergetic analyses to investigate the effect of the design, as well as the important operating parameters that account for the efficiency of a PEM electrolyzer plant. This study defined how much energy efficiency decreases with increasing operating temperature, decreasing current density, reducing electrolyte thickness and increasing electrode catalytic activity. In addition, F. Sorgulu & I. Dincer [15] studied a combined system consisting of a steam and gas turbine integrated with solar energy, to which they added an electrolyzer, to provide a sustainable energy resource based on hydrogen; through thermodynamic analysis, the different alternatives to increase the energy and exergetic efficiencies of the system were, in general, known. Moreover, with the help of studies related to exergetic efficiency, A. AlZahrani et al. [16] analyzed several operating conditions, state properties and the optimal production of hydrogen, using a model based on a solid oxide electrolyzer cell (SOEC).
As mentioned above, the increasing use of fossil fuels has caused serious problems for the environment [6]. Therefore, different countries have shown interest in making studies related to the environmental impact and emissions of fossil fuels, mainly those related to internal combustion engines. For this matter, the proposal of dual-fuel engines that use more environmentally friendly fuels was born; as a result, Da Costa et al. [17] evaluated, both theoretically and experimentally, the performance characteristics of an engine that operates dually using natural gas and diesel, performing energy and exergetic analyses and finding that both the energy and exergetic efficiencies increase when operated in this way. Moreover, other authors [18] have studied the performance of an engine operating only with alternative fuel, such as ethanol, in which the efficiencies of the first and second laws were evaluated as functions of engine speed, engine load and air-fuel ratio. O. Balli et al. [19] studied the effect of hydrogen fuel on the exergetic performance of a turbojet engine used in a military training aircraft, where the results indicated that the use of hydrogen seriously affects the exergetic performance of the engine and its components. However, the exhaust emissions to the environment decreased significantly.
Based on the above, the importance of integrating hybrid systems to achieve better overall performance is evident and thus, it is proposed to implement an integrated system consisting of a dual-fuel engine (diesel, hydrogen and air), a thermoelectric generator and an electrolyzer, which takes advantage of the high exhaust gas temperatures from the engine. Next, a thermoelectric generator obtains electric energy that is then used by a PEM electrolyzer to produce hydrogen that serves as a partial replacement of the engine’s fuel. However, the work potential of this system is unknown, i.e., the maximum useful work that can be obtained from the system without violating thermodynamic laws, as well as the best performance that such systems can deliver [20]. In addition, in some cases, it is necessary to develop mathematical models that complement the thermal models to allow to predict the behavior of the analyzed systems [21,22]. Consequently, for future validation, the use of test stands is required since they are easy to use and provide extensive applications for research [23]. Therefore, this article aims to determine the optimal operating conditions of each subsystem by performing destroyed exergy and exergetic efficiency studies using thermodynamic models based on the second law, which is then compared with various references for support and validation. Once positive results have been obtained, the behavior of each subsystem in the references is analyzed by varying the parameters that influence their performance.
2. Methodology of thermodynamic modeling of each subsystem
According to the previous bibliographic references, the exergetic analyses have played a key role in the evaluation and improvement of the performance of a system, since by using them it is possible to identify the elements that exhibit higher irreversibility, to quantify them and to identify the sources that generate them [24]. In this regard, by studying the second thermodynamic law, opportunities are revealed to minimize the irreversibility and to maximize the global performance of any technical system, contrary to studying the first thermodynamic law, which only allows quantifying the energy during a process and does not offer opportunities for improvement [25].
For this reason, this article is based on the principles of the second law of thermodynamics to obtain relevant information on the behavior of each sub-system, such as its exergetic efficiency and destroyed exergy and validating each model with bibliographic references.
2.1. System description
For this study, the basic functioning of three sub-systems was taken into account. In order to do the exergetic analysis of each system, it was necessary to take into account the mass and energy balances on the respective control volume.
Table 1 shows the measuring instruments used for each subsystem in order to know the variables involved.
Table 1 Instruments used for measurements
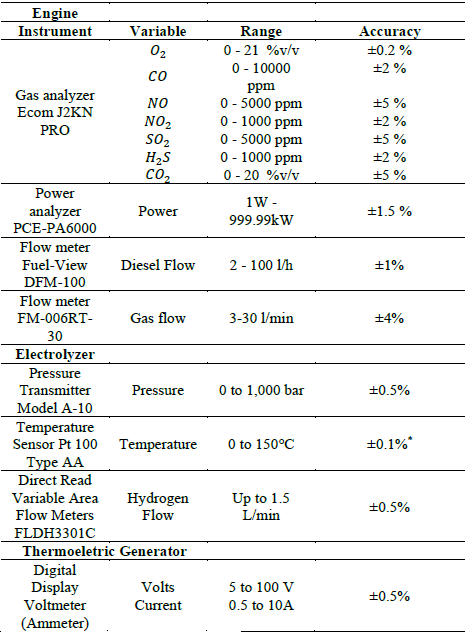
*The industry standard accuracy at 0°C
Source: The Authors.
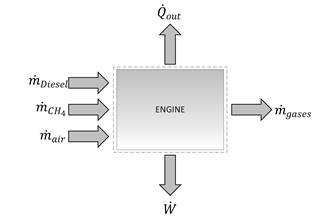
Initially, a dual-fuel engine is considered that works at different powers. The description of the theoretical model is shown in Fig. 1, where the mass flow of diesel and methane is considered to enter the engine and mix with a mass flow of air; the mixture burns completely and the combustion products leave the engine. The engine produces an output power and transfers an amount of heat to the environment.
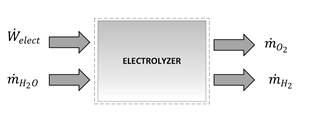
Next, there is the theoretical model of a PEM electrolyzer, as shown in Fig. 2, having as inputs a constant electric current and a mass flow of water in the subcooled state, as well as output products that are decomposed by electrolysis, such as hydrogen and oxygen.
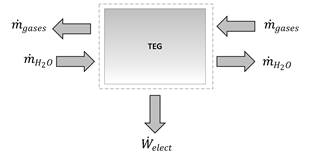
Finally, there is a thermoelectric generator that is a shell and tube heat exchanger with tubes covered with thermoelectric materials. The theoretical model of this subsystem, shown in Fig. 3, considers that the hot side is fed by the mass flow of hot flue gas coming from a turbine passing around the tubes, while the cold side is fed by seawater pumped by a circulating pump that flows inside the tubes. Since there is a temperature difference between both sides of the TEG, electricity is generated.
The integrated system proposed by the authors aims to harness the potential energy from the exhaust gas stream of a dual-fuel engine, used as a hot fluid for a thermoelectric generator whose cold side is fed by water. This temperature differential will produce the electrical energy needed by an electrolyzer to obtain hydrogen that is then used as part of the fuel of the engine under dual operation. The studies mentioned above are intended to increase the overall efficiency of this system.
3. Thermodynamic model
The exergetic analysis applied to each sub-system was based on the second law of thermodynamics.
3.1. Exergetic analysis
For every sub-system, an exergetic analysis was performed by varying different input parameters, taking into account the following considerations:
Every sub-system is in a stable state
Sub-systems are treated as open systems and the reference state was defined as T_0=293 K and P_0=1 atm.
Exhaust gases were treated like air with an ideal behavior
The mass flow of exhaust emissions from the engine is equal to the sum of the mass flow of diesel fuel, methane and air entering the combustion chamber
The air entering in the combustion chamber is at ambient temperature and pressure, so it does not provide exergy to the sub-system
The potential energy and kinetic effects of the flow of incoming and outgoing fluids are neglected
Hydrogen and oxygen are treated as ideal gases
In the electrolyzer, the exergy of water and oxygen is neglected
The exergy balance in a control volume can be generally expressed in terms of a ratio given by eq. (1):
where the net energy transfer rate can be given by heat, work and mass, as expressed in eq. (2)-(4) :
The specific physical exergy was calculated according to Eq. (5):
For a compressed liquid, assuming the enthalpy h is given for low and moderate pressures and temperatures, it is possible to considerably reduce the error when evaluating ℎ by:
Because hydrogen and oxygen were treated as ideal gases, their physical exergy is calculated by:
The specific chemical exergy for diesel is given by Eq. (8):
where H u is the net caloric value and φ is the chemical exergy factor, which, for liquid fuels, the effect of sulfur was included in the correlation according to the expression in [26]:
where h,c,o and ∝ are the mass fractions of H, C, O and S, respectively. The accuracy of this expression is estimated to be ±0.38%.
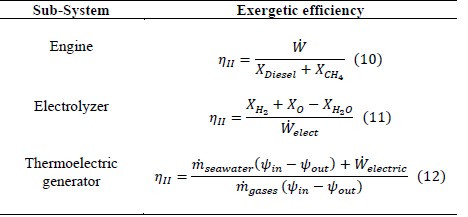
The efficiency of the second law for each subsystem is shown in Table 2.
3.2. Validation
According to the previous studies related to the second thermodynamic law behavior of dual combustion engines, the thermoelectric generators and electrolyzers allow comparison and validation between the models proposed by each author and those presented in this article. Therefore, what has been proposed is validated.
3.2.1. Dual combustion engine
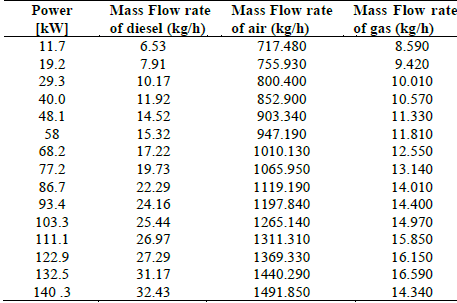
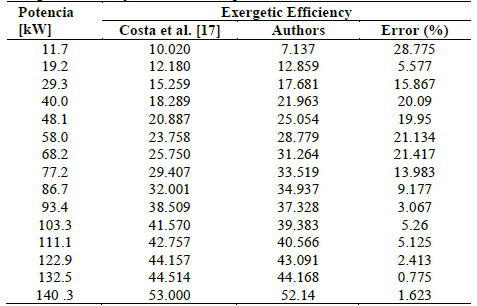
Ramos da Costa et al. [17] studied an electro-mechanical system composed of a commercial engine (Cummins 6CTA) with mechanical power of 188kW at 1800 rpm, equipped with air, gas and diesel flow meters, temperature and pressure sensors at different points making it ideal for gas analysis. For this, it is assumed that the air enters at standard conditions and therefore does not contribute exergy to the system. Similarly, fuels (diesel and natural gas) enter at ambient pressure and temperature, so the exergy provided by these amounts only to specific chemical exergy. The data in Table 3, provided by the author, is considered.
Costa et al. [17] varied the engine power in a range between 10 kW and 150 kW, with increments of 10 kW and different mass flows of diesel fuel and methane for each variation.
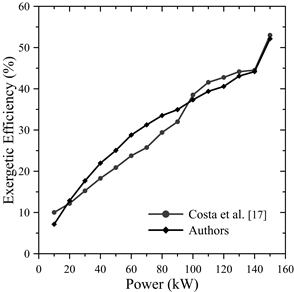
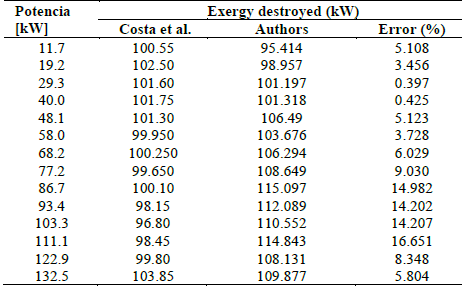
Fig. 4 shows the behavior of the exergetic efficiency as a function of the engine power, where it is evident that for higher power values, the second law efficiency improved significantly. When increasing the load from 80 kW to 150 kW, this efficiency increased by 55.55% until reaching its maximum value; this is due to greater use of the exergy contributed by the fuels. The highest error calculated was 28.775% with respect to the data revealed by the study of the previous author, as shown in Table 4.
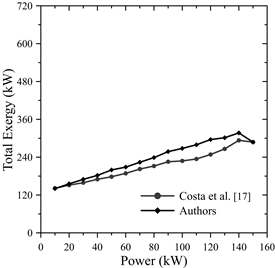
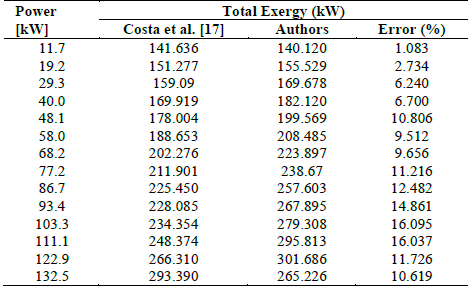
As the load on the engine increased, the total exergy input increased, as shown in Fig. 5. From 80 kW to 150 kW, the total exergy input increased by 17.04%. The calculated data revealed a maximum error of 16.1% compared to the data provided by Costa et al. [17], as shown in Table 5
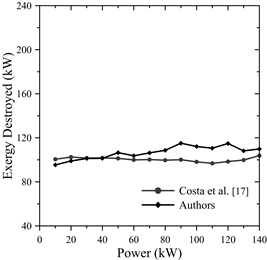
Moreover, they calculated the exergy destroyed for different powers. This resulted in Fig. 6, where it is observed that the exergy is approximately constant, i.e., the exergy shows few variations when altering power-which is congruent with the results in this article-and reveals a maximum power error of 31.217%, as shown in Table 6.
3.2.2. Electrolyzer
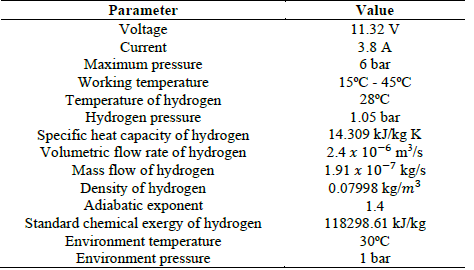
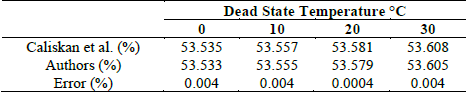
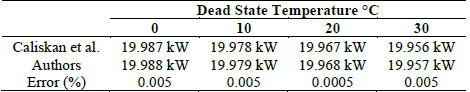
In order to validate the electrolyzer, the results obtained by Caliskan et al. [27] were taken as a reference, in which an exergetic analysis was developed for a PEM electrolyzer with 10 cells. The parameters used in the case studies are shown in Table 7.
Using Figs. 7 and 8, it was observed that the results of the reference model show congruence with those proposed in this article, with an error of 0.004% and 0.005%, respectively. Tables 8 and 9 show the results obtained in this article and those in the reference.
3.2.3. Thermoelectric generator
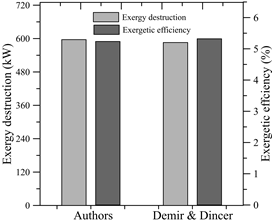
In order to validate correctly the exergetic analysis performed on this subsystem, the results of Demir et al. [28] were taken into account, where an exergetic analysis of a hybrid cogeneration system for electricity and freshwater production was performed. The system consists of a solar-natural gas hybrid power plant, a thermoelectric generator (TEG), a Rankine cycle to produce electricity and a flash distillation unit to produce fresh water. The TEG unit is used to take advantage of the gases produced by the gas turbine, where tin sulfide (SnS) and bismuth telluride (Be2Te3) is the selected thermoelectric materials for the TEG. The parameters considered were 100kPa, 25°C for the atmospheric conditions and a gas turbine flow rate of 42.26 kg/s. The hot side of the TEG is fed by flue gases at 227°C and 100 kPa, which then leaves the TEG at 189°C and the same inlet pressure. In addition, the cold side of the TEG is fed by seawater pumped by a circulating pump at 20°C and exits the TEG under slightly different conditions. For that reason, the exergy of the cold side of the TEG is neglected.
According to the parameters above, the destroyed exergy and the efficiency were calculated and plotted in Fig. 9.
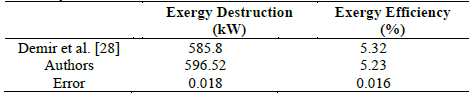
By comparing the results of the models, it follows that, as shown in Table 10, the error rate for both study cases is approximately 2% and thus, it is within the allowed range.
Table 10 Comparison of values obtained for the exergy destruction and exergy efficiency
Source: The Authors
In both cases, it is possible to show that the behavior is congruent since by obtaining higher exergy destroyed using the model proposed in this article, it was expected that the exergetic efficiency was lower than that of Demir et al.
4. Results and discussion
For each sub-system, a case study was applied by varying different parameters and analyzing the results obtained.
4.1. Case study I: Dual-fuel engine
In the dual-fuel engine, the behavior of the exergy destroyed and the exergetic efficiency as a function of methane concentrations for three different power values was analyzed using their respective fuel mass flows. Initial concentrations were 10% methane and 90% diesel, increasing the concentration of methane by 10 percent. This resulted in Fig. 10, where it was observed that the exergy destroyed is directly proportional to the concentration of methane; moreover, it can be inferred that more exergy is destroyed when the power or the slope is increased, i.e., at higher power levels, the sensitivity to destroy exergy is greater with increasing methane concentrations.
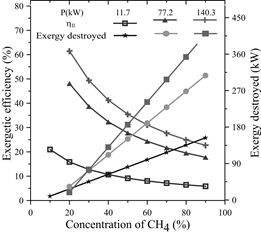
Source: The Authors.
Figure 10 Exergetic efficiency and Exergy destroyed as a function of the concentration of CH 4 .
For the lower power level, for every 10% increase in methane concentrations, the exergy destroyed increases by 18.016 W. For the medium power, it increases by 39.166 W and for the highest power, the exergy destroyed increases by 55.728 W.
On the other hand, the exergetic efficiency decreases whenever the mixture contains higher levels of methane; however, for higher power levels, the exergetic efficiency increased. For the studied interval of CH4 concentrations, the exergetic efficiency decreased by 72.10% for 11.7 kW, varying from 5.85% to 20.97%; for 77.2 kW, the exergetic efficiency decreased by 63.08%, varying from 17.76% to 48.12% and, for 140.3 kW, the exergetic efficiency decreased by 63.08%, varying from 22.69% to 61.46%.
4.2. Case study II: Electrolyzer
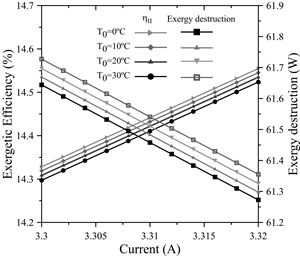
The input exergy was modified in the electrolyzer by modifying the input current from 2.2A to 5A in increments of 0.4A and keeping the voltage constant at four different reference temperatures.
The results are shown in Fig. 11, where it is observed, on a macroscopic scale, how the exergetic efficiency potentially decreases for every change in current levels. Thus, for 2.2 A, the efficiency reached a maximum of 92.5915% and, for 5A, the efficiency decreased by 51.8%, reaching 40.74%. This happens because by supplying a higher current during the electrolysis process, the input exergy to the electrolyzer increases, whereas by keeping the output exergy constant, the exergetic efficiency will be lower; this is consistent with the research done by Esmaili et al. [29], as well as Meng Ni et al. [14].
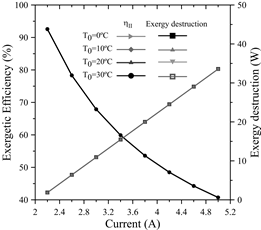
Source: The Authors
Figure 11 Exergetic efficiency and Exergy destruction as a function of the current.
Moreover, the exergy destroyed increased linearly as a function of the current; this means that for every 0.4 amperes of input current, the exergy destroyed increased by 4.53 watts.
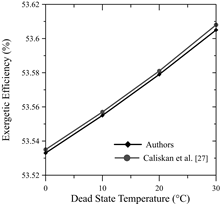
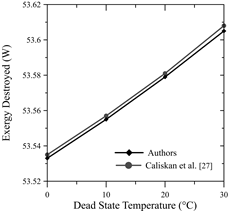
Meanwhile, for every 10°C decrease in the dead state temperature, the destroyed exergy increased by approximately 0.1%. In addition, the above-mentioned authors also agree with an increase of the irreversibilities at greater current levels. This is due to the activation overpotential of the anode, cathode, as well as the electrolyte’s ohmic overpotential. The ohmic overpotential close to the proton-exchange membrane is related to the resistance of the membrane to hydrogen ions crossing it. The ionic resistance is a function of humidification, thickness and temperature of the proton-exchange membrane. In addition, the activation overpotential corresponds to the energy barrier that has to be overcome in order to start a redox electrochemical reaction, especially those related to the electron transfer that happens at the electrode interface [30].
Fig. 12 shows the behavior of the exergetic efficiency and exergy destruction for a shorter input current interval. This allows to observe the changes whenever the reference temperature is modified, for this is done on a smaller scale.
4.3. Case study III: Thermoelectric Generator (TEG)
In the TEG, the behavior of the exergetic efficiency and the exergy destroyed was analyzed by varying the flue gas inlet temperature from 470 K to 560 K in 10 K increments, for mass flows of 32.26 kg/s, 42.36 kg/s and 52.36 kg/s. The results of this analysis are shown in Fig. 13, where the behavior of the exergetic efficiency as a function of the different exhaust gas temperatures, as well as the destroyed exergy, is observed.
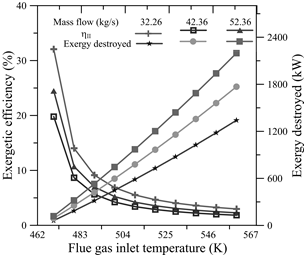
Source: The Authors
Figure 13 The variation of the exergetic efficiency and the exergy destroyed by changing the flue gas inlet temperature.
For the three mass flows analyzed, 86.68% of the decrease in the exergetic efficiency occurred between 470 K and 510 K; this means that from 510 K, the exergetic efficiency values showed minimal variation.
Moreover, the destroyed exergy displayed a linear behavior for changes in flue gas temperature. An increase in the mass flow of flue gases also produced an increase in the destroyed exergy. For the lowest temperature analyzed, this resulted in increases of 45.63% and 31.33% for every variation in the mass flow, while for the maximum temperature analyzed, the increase was 31.95% and 24.21%, respectively.
5. Conclusion
The exergetic analysis is a fundamental tool at the time of evaluating the performance of a system, as it allows to quantify the irreversibilities of a process, make comparisons and identify opportunities for improvement. Therefore, it is very useful to apply these analyses to hybrid systems when it comes to evaluating their thermodynamic behavior. In order to know the useful work and the efficiency that the system proposed in this article would have (dual-fuel engine - TEG - electrolyzer), it was necessary to do this type of analysis where the exergetic models of each subsystem were compared and their reliability was determined through validation using different references, resulting in average errors of around 10% for the engine, less than 0.01% for the thermoelectric generator and less than 0.02% for the recuperator. It was also observed that for a dual fuel engine (diesel and natural gas), varying the concentration of methane from 10% to 90%, for the power of 11.7 kW, caused a decrease in the exergetic efficiency of 72.10%, whereas, for 77.2 kW and 140.3 kW, this resulted in a decrease by 63.08% in both cases. In addition, for every 10% increase in methane concentrations, the destroyed exergy increased by 18.02 kW for 11.7 kW, 37.17 kW for 77.2 kW and 55.73kW for an operating power of 140.3kW. The latter results also show that the exergy destroyed is greater when the operating power of the engine increases.
In the case of the electrolyzer, it was observed that an increase in the input current significantly affected the exergetic efficiency of the electrolyzer: as the input current varied from 2.2A to 5A, for reference temperatures of 0ºC, 10ºC, 20ºC and 30ºC, the exergetic efficiency decreased by 56% in all cases. The latter is to be compared with the destroyed exergy, which increased notably by 94.41%, 94.44%, 94.47% and 94.50%, respectively, as a result of the variation of the above-mentioned parameters. On the other hand, for the TEG, it was noticed that the exergetic efficiency decreased whenever there was an increase in the flue gas inlet temperature; the most significant changes occurred from 470 K to 510 K, as this amounted to 86.68% of the total decrease. Furthermore, the destroyed exergy increased linearly by increasing the flue gas inlet temperature, as well as by modifying the mass flow of the fluid. For mass flows of 32.26 kg/s, 42.36 kg/s and 52.36 kg/s, the exergy destroyed ranged from 61.64kW to 1382.10kW, 89.77kW to 1769.29kW and 117.90kW to 2197.71kW, respectively.
This study gives a clear idea of the optimal operating conditions of the studied sub-systems. In future works, it is intended to integrate them and to reach high global performance.