1. Introduction
Polyhydroxyalkanoates (PHAs) are biodegradable bioplastics that belong to the family of polyesters. They are synthesized as an energy reserve by microorganisms, especially bacteria [1], during limited conditions of some essential nutrient, for example nitrogen, phosphorus or oxygen, they can also occur when carbon is present in excess. [2]. The kinetics of PHAs accumulation can be divided into two groups, in the first one the production of PHAs is induced by limiting nutrients such as nitrogen combined with an excess in the carbon source, while in the second group, production occurs throughout the growth of the bacteria, i.e. growth-associated production [3].
PHAs are classified into 3 groups according to their of monomer side chain; Short chain length, scl (5 or less carbons), medium chain length, mcl (6 to 14 carbons), and long chain length, lcl (more than 14 carbons) [4]. PHAs monomers have applications in various biochemical products and their hydroxymethyl fatty esters can be used as biofuels [5]. The monomeric composition depends on the carbon source and the microorganism used [6] and can be manipulated to alter its physical properties based on its final purpose by modifying operational conditions, such as the proportions of substrates used, [7], or the feeding rate [8]
However, despite all its advantages, high production costs (compared to petrochemical plastics) remain the biggest obstacle to the industrial production of PHAs. These costs are mainly incurred by the carbon source, the fermentation process and the purification. Even so, there are ways to reduce them, e.g. using low-cost carbon sources and increasing volumetric productivity [9,10]. In relation to the above, the different production systems must be considered. The batch is the simplest way to operate; the medium and the carbon source are added at the beginning of the process whose exahustion marks the end of the process and despite its simplicity, it requires time to start again the cycle and and because the substrate concentrations are changing, the growth rate and product formation cannot be maintained for long periods of time, limiting its productivity [11]. Therefore, if more polymer is required to be produced, continuous or sequential feeding of medium must be added, if this is done without removal of liquid media, the system is considered a fed-batch [12]. This system is the most used at the industrial level to produce PHAs, since it allows to generate high cellular concentrations and polymer accumulation allowing the high production, but due to its limited workload, it requires feeding concentrated substrates [13,14]. Furthermore, there are also other continuous operational modes such as production in single-stage or multi-stage systems, immobilization and recirculation. [15]. However, different limitations related with this type of systems should also be considered, e.g. the risk of contamination of the reactor or the back-growth in the reservoir of the feeding medium, the risk of cell washing when the dilution rate (D) approaches the maximum growth rate (µmax) and the challenges of preserving the genetic stability of the strains [13].
This review aims to determine the limitations in the production of PHAs based on the substrates used, the type of polymer to be produced and the metabolic characteristics of the microorganism used, in order to provide a better understanding about its advantages and disadvantages and thus contribute information to future research that allows to improve the cultivation strategy used according to the mentioned criteria.
2. Materials and methods
A systematic search protocol for scientific literature was conducted for this study, based on the prism statement [16]. Indexed databases widely used in the scientific field: Science Direct and Scopus, and a non-indexed database, Google Scholar, were accessed. The search criteria were based on the question: What limitations are presented in the culture strategy used to obtain PHAs in terms of the characteristics of the substrate, the type of polymer or the chosen bacteria?
Original articles in English focused exclusively on the PHA production, published in the last 10 years in indexed journals located in the first two quartiles (Q1 and Q2) were taken into account the microorganisms were bacteria and it is specified the productivity obtained with the system (or it can be calculated) and the type of PHA produced, and optionally kinetic parameters such as: the growth rate of the microorganism, the percentage of biopolymer accumulation and the yield of the substrate product. And articles about photosynthetic bacteria or solid-state cultures or mixed cultures were excluded.
Additionally, several criteria were taken into account such as sensitivity, by including Health Sciences Descriptors (DeCS descriptors) in the search path, specificity when using Boolean connectors and completeness when using non-DeCS descriptors and including articles from a non-indexed database to complement the information. Finally, the general search path was as follows: bacteria AND (pha OR phb OR phv) AND (batch OR fed-batch OR continuous)) (See Table 1). Subsequently, the Mendeley ® Desktop library manager version 1.19.3 was used to organize the results and remove duplicate articles.
3. Results
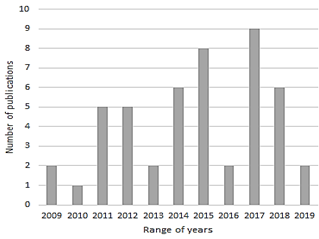
A total of 442 publications (Science Direct 44 and Scopus 398) were obtained (Fig.1), 34 duplicate articles were removed using Mendeley ® Desktop, the remaining 408 articles were evaluated based on the title and abstract, verifying agreement with the inclusion criteria mentioned above. In this step 346 articles were deleted, and the remaining 59 articles were read in full text, finally 48 were kept according to the defined exclusion criteria. Furthermore 15 publications were subsequently added as part of the exhaustiveness criteria, making up a total of 63 publications included in this systematic review.
4 Discussion
4.1. Distribution of the articles found
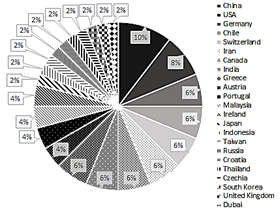
There is an increasing trend in the number of publications over time (Fig. 2), which may indicate an increase in the international community's interest in the production of this type of biopolymer. These polymers represent a sustainable alternative to petroleum-derived plastics, which increasingly accumulate in the environment at such a high level that it is estimated that 79% of the world's plastic production ends up deposited in nature, generating a negative impact on wildlife, natural environments, and human health. Currently, the industrial scale production of these bioplastics is already being done by companies such as KANEKA Corporation (Japan), Biomer (Germany), Bio-on (Italy) and Meridian Inc. (USA) [17]. Due to the growing market of bioplastics, more countries are expected to conduct research on the subject. In this review, publications from 23 different countries were found, with China and the United States leading the way with 10% and 8% of publications found respectively. (Fig. 3).
4.2. Distribution of the types of PHAs produced
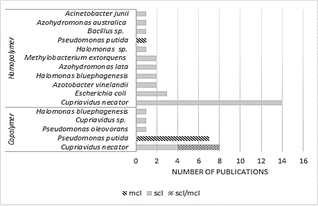
Fig. 4 shows that the majority of articles reported the production of homopolymers and the rest corresponds to copolymers. The scl-PHAs represent 75% (36 publications) and the mcl-PHAs 16.7% (8 publications). This lower percentage could be due to the fact that more expensive substrates are required and lower yields are obtained compared to scl-PHA. Besides Its monomeric composition is difficult to standardize, which may cause variations in the properties of the final product [18]. These reasons could also explain the low number of homopolymer articles of the mcl type. Most of the mcl were reported in the form of scl-mcl copolymers and hybrids. These kinds of hybrids allow the improvement of processing properties such as melting temperature (Tm), crystallinity and flexibility, which resemble those of low-density polyethylene, including tensile strength, modulus of Young and the elongation at break and that improve the higher the proportion of mcl [4, 19]. On the contrary, lcl-PHAs were not found in this review. The latter are rarely described in the literature and are only found forming copolymers with PHAs of the scl or mcl type [20].
4.3. Distribution of the modes of operation used in the production of PHAs
In general, the fed-batch system was the most reported (32 publications), followed by the batch system (11 publications) and finally the continuous system (5 publications) (Fig. 5). 12 cultures were reported growth-associated and 36 publications were reported non-growth associated. Consistently with this majority, most of the cultures were carried out in fed-batch. This system generally uses 2 stages; one first phase of growth to obtain high cell density cultures and a second phase designed to promote the accumulation of PHAs [13].
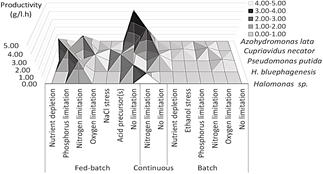
Source: The Authors.
Figure 5 Visualization of maximum productivity (Y axis), for the 5 species with the highest productivity found (Z axis), categorized by type of system and induction used (X axis)
Additionally, is observed that the productivity obtained from the batch systems is considerably lower than in the other modes of operation, this is appreciable in Table 2 since no reported batch system appears. The above can be explained because in this last system there is no subsequent addition of nutrients, therefore cell density and productivity are limited. These systems are more useful for the physiological compression of the microorganism, but if you want to obtain greater productivity, you must add nutritious food either in a batch-fed or continuous mode, coupling the system to the polymer formation characteristics and the nutritional requirements of the microorganism. In these systems, productivity is improved due to the fact that concentrated nutrients can be added in a small volume, which allows to increase the cellular concentration and the polymer obtained. [13].
Table 2 Cultivation details and parameters for publications with reported productivity ≥ 1 g/l.h
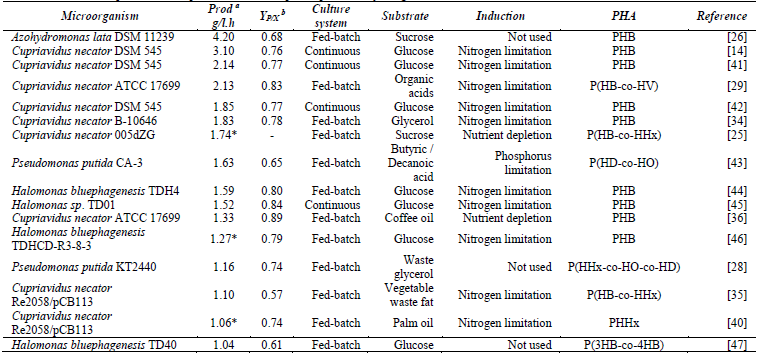
(a) Volumetric productivity. (b) Product yield in biomass. g g-1. (*) Indirectly calculated values based on the polymer concentration (g/l) over the operating time (h) reported in the respective reference.
Source: The Authors.
On one hand the fed-batch system is widely used on a pilot and industrial plant scale, it should be considered that the reactor volume and the operating time become limiting factors due in large part to the preparation and post-treatment required to start the bioreactor in each cycle. On the other hand, the continuous cultivation system reduces downtime for the restart of the production process and additionally, helps to reduce investment costs by requiring smaller facilities, allowing to obtain volumetric productivity similar to or greater than those of a fed-batch system [21].
Despite the above, the best productivity was generally found in fed-batch culture, however, it should be taken into account that there were only 5 publications with a continuous cultivation system, as opposed to 36 in the fed-batch system. Consequently, it is notable that the second-best productivity reported was in a continuous system. An explanation for this low number of publications may be that, most bioprocesses are more likely to become contaminated when operated for long periods of time, this becomes a major obstacle for these systems to be used on a large scale, however, strategies that minimize or exclude the growth of unwanted microorganisms can be applied, for example, by integrating nutrient and feast-famine cycles into the process, where low concentration of nutrients during the cycle only would allow the growth of PHA-storing microorganisms, because PHAs act as an energy reserve [13]. Another alternative is to attach a sterilization system of the feeding medium by filtration, although in this review, this method was more common in substrates pretreated by other microorganisms [9,22].
Another reason that explains the low number of publications with a continuous system is that the great majority of the reported production was carried out with microorganisms that produce PHA in a manner not associated with growth (Fig. 5). This characteristic influences the use of this system because the PHA not being related to growth and being intracellular depends on the biomass that is previously formed, so it requires separate stages of growth and accumulation, which would not be possible to perform in a Continuous system with a single reactor, since in the accumulation stage, there would be a washing of biomass because there would be no cell renewal, however, it would be possible to attach several cascade reactors that would allow the stages to be separated. [23]. On the contrary, a single reactor system would be appropriate for microorganisms whose polymer accumulation is not related by growth, since growth and accumulation would occur simultaneously and a single reactor could be carried out [24]. Therefore, more research should be carried out in this field, since as mentioned, this cultivation system would improve productivity and competitiveness.
4.4. Distribution and limitations of the types of substrates used in the production of PHA
In Fig. 6, the peaks of maximum productivity are visualized according to the type of substrate and microorganisms used, organized from higher to lower productivity. Saccharides were the most reported type of substrate as well as the most used in different microorganisms. The following are some of the limitations found associated with the substrates used in the PHAs production:
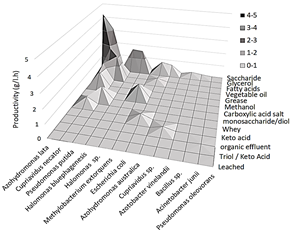
Source: The Authors.
Figure 6 General illustration of the best productivities (Y axis) by species (X axis) and type of substrate (Z axis).
Saccharides: These substrates come mainly from industrial effluents and can be used as low-cost substrates, but some have low carbon concentrations, for example, whey (45 g / L lactose) or lignocellulosic hydrolysates, which are not appropriate for direct use in the industrial production of PHA in batch systems, due to the low cellular densities that are obtained [14]. This occurs when a low concentration of substrate is fed, the culture media are diluted, decreasing their concentration [25]. This, together with the limited volume of a batch feed system, makes it unsuitable for this system. Evaporation process is an alternative with the purpose of concentrating low concentration substrates, but additional costs would be generated in the process and additionally, the toxic inhibitor components such as hydroxymethylfurfural and furfural present in these substrates could be concentrated [14]. Regarding the type of PHAs, the results show that all strains that produced scl-PHA type polymers use sugars as a substrate for accumulation, with the exception of a strain of Cupriavidus necator harboring Escherichia coli W csc genes which produced poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) due to genetic modifications [25]. The maximum productivity (4.2 g / l.h) found with sugars was reported in sucrose by Azohydromonas lata [26].
Fatty acids: Most of their limitations are associated with their low tolerance and high toxicity, mainly due to the alteration of the membrane potential and the transport of electrons in the cells [27]. This toxicity makes not viable the production of PHA in systems such as batch. One way to overcome this is to use poorly soluble fatty acids such as oleic acid [28]. Another solution is to use a fed-batch system since it can be dosed to the ranges tolerated by microorganisms [29]. This feed can be optimized by online measurement methods, e.g. attached to O2 and CO2 concentration, which allows the substrate to be supplied when microorganisms really require it [30]. Another common limitation is the high price of some substrates such as nonanoic acid. As an alternative, the bioprocess can be performed in two stages; the initial one with some sugar as a substrate for growth and in the following stage add the substrate when there is a certain level of biomass (accumulation phase) [31]. This strategy was used at a pilot-scale to obtain mcl-PHAs from Grape Pomace supplemented with fatty acids during accumulation phase [18]. This type of substrate was present in all articles that reported production of mcl-PHAs or mcl/scl hybrids, but the highest productivity found on this substrate (2.13 g/l.h) was reported by Cupriavidus necator DSM 545 producing a scl-PHA [14], corresponding to the second highest within this review.
Glycerol: This substrate is mainly obtained as waste from the biodiesel industry. In the production of PHA it has been reported that it can cause growth inhibition due to methanol and sodium ion residues. However, this problem can be avoided by controlling the maximum tolerable concentrations by the microorganism. [32]. It has also been reported that when using glycerol as a substrate for the production of PHA, the polymer composition could be altered due to end-capping (esterification at the end of the PHA chain) producing a slight decrease in tensile strength and in Young's module [33]. It was found that in all 4 articles in which it was used as only substrate, PHB was always produced, between these, the highest productivity (1.83 g/l.h) was reported by Cupriavidus eutrophus (C. necator) B-10646 using high-purity glycerol at pilot scale [34].
Fats: The main limitations of this substrate are foam generation that may cause problems due to the loss of biomass and the increased risk of contamination. Nevertheless, adding more headspace in the fermenter and the use of foam centrifuges could counteract it [4]. Another issue is the possible solidification of the fats in the feed lines; however, this problem can be solved with heating and rapid pumping to avoid substrate cooling [4]. Another common limitation that arises is the low bioavailability of the substrate, due to its immiscibility with the culture medium and the uneven accumulation and distribution. An alternative solution is to use compatible surfactants [35] such as free fatty acids, which additionally make the triacylglycerides of the fats more susceptible to the action of the lipases of the microorganisms, improving the metabolization [36]. This substrate was reported in 2 articles from different countries, using the same strain (C. necator Re2058/pCB113) and the same type of polymer P (HB-co-HHx) was obtained [4,35], between these, the highest productivity (1.1 g/l.h) was achieved in a fed-batch cultured in a 5L bioreactor (working volume 2 L) using sludge palm oil, a residual palm oil by-product [35].
4.5. Distribution of articles by species and reported productivity
Despite the diversity of microorganisms that were found, only 5 species distributed in 16 articles stood out for obtaining productivity greater than or equal to 1.0 g/l.h whose general information is shown in Table 2 in order from higher to lower productivity. Cupriavidus necator was the species with the most publications in the last 10 years, followed by Pseudomonas putida, and Escherichia coli. However, Azohydromonas lata reported the highest productivity found (4.20 g/l.h), despite being present only in 2 publications (Fig. 4), followed by C. necator with 3.1 g/l.h and then P. putida with 1.63 g/l.h (Fig. 6). On the other hand, E. coli was third in the number of publications (Fig. 4) but it was not noted for its productivity, actually, it was the seventh in productivity by species (Fig. 6 ) this could be due to several reasons; to begin with, E. coli does not naturally produce PHAs but requires genetic modifications such as the insertion of Cupriavidus spp. genes to synthesize P (3HB), this can lead to lower efficiency limitations of the heterologous route of PHB production compared to a route natural [37].In the study of [38] in which the maximum productivity for E. coli was reported (0.75 g/l.h), there were problems of growth inhibition due to toxic metabolites (xylitol phosphate) from the heterogeneous route of xylose utilization and the additional metabolic burden of overexpressing foreign proteins.
4.5.1. Cupriavidus necator
This bacterium is the most studied in the production of PHAs, since it is easy to grow, is not pathogenic for humans and it grows rapidly in simple sugars, additionally it can accumulate up to 90% dry polymer weight, and has the capacity to produce it in growth-associated and not associated [24]. The two highest productivity in this species were reported using glucose as a substrate (Table 2) and the third-largest using organic acids as a substrate (Fig. 6). In the latter study, the addition of propionic acid allowed the copolymer to be obtained. P (HB-co-HV), a scl that has the advantage of having better thermal and mechanical properties than PHB, and was performed in high-density fed-batch culture. In this case, the use of this culture system allowed avoiding the toxic effect generated by organic acids, which must be in concentrations below 1 g/l for acetic acid and 0.5 g/l for propionic acid [29]
Regarding the type of PHA produced by C. necator, scl was the most reported. Within the enzymes involved in the synthesis of PHAs, 4 classes of PHA synthase are reported, of which 3 (I, III and IV) can synthesize this type of polymer, and C. necator, possesses class I, performing the biosynthesis from the acetyl-CoA obtained from glycolysis, which in turn is converted to acetoacetyl-CoA, then to 3-hydroxybutyryl-CoA, and finally to the mcl, respectively by the enzymes β-ketothiolase, acetoacetyl-CoA reductase, and PHA synthase [39]. Even so, C. necator was found to produce scl-mcl hybrids, in 4 studies, but in all of them, they used mutant strains of Cupriavidus necator H16 with the ability to produce PHHx [4, 25, 35, 40]. The strain that obtained the highest productivity in these 4 studies (1.74 g/l.h) (Table 2), has the particularity of carrying Escherichia coli W csc genes in order to assimilate sucrose.
Regarding the cultivation systems used, C. necator reported the best productivity (3.1 g/l.h) [14] in a continuous (semi) system with recirculation of biomass generating 148 g/l of biomass. On one hand this cell-recycle mode of operation was the only one reported in this review and the same authors indicate that it has been little used in the production of PHAs. Cell recycle was performed with an external microfiltration module which extracts biomass and discards a cell-free permeate. On the other hand, the second highest productivity in continuous systems in this review was 2.14 g/l.h [41], with a biomass concentration of 81 g/l. The continuous system used in this study consists of 5 reactors operated in cascade, where the first 2 are used to promote cell growth and the last ones are used to induce polymer production by limiting an essential nutrient, this way the system allows improving the productivity of the process. This strategy is useful for non-growth associated producers or in this case partially associated. Considering that both systems used the same strain (Cupriavidus necator DSM 545), achieving a similar accumulation percentage of PHA between both studies (Table 2), it can be deduced that the higher productivity obtained with the recirculation system can be attributed to the high-density strategy and the dilution rate (D) used in the culture. In continuous systems, this parameter can be directly related to the specific growth rate (µ) of the microorganism. When comparing this parameter between the two strategies, in the cell recycling system, a dilution rate of 0.24 h-1 was used, which is higher than the total dilution rate used in the 5-stage reactor that was 0.034 h- 1, and although the operation at high dilution rates can produce a washing effect in classical continuous methods, when cell recycling is used not only this effect is avoided, but in fact it allows to increase cell density and therefore the productivity of the process. [15].
A high cell density culture is the one that reaches biomass concentrations higher than 100 g/l [48]. This is a requirement to obtain high productivity [43] and additionally it was another differentiating element between both systems. In spite of this, (Horvat et al., 2013) [41] reports that through the optimization of the mathematical model that represents the cascade system, a theoretical productivity of 9.95 g/l.h could be achieved, after getting over some limitations such as the fact that the physiological state of the cells is different in each reactor so it’s difficult to predict them in each one, and during an excessive time in the limitation of nitrogen, the constitutive and inductive enzyme systems that catalyze the synthesis of PHA could be altered, decreasing productivity in consequence. In addition, another important obstacle described is the difficulty in maintaining an OTR (Oxygen Transfer Rate) sufficient to reach high cell density cultures, in addition to the fact that C. necator can produce exopolysaccharides which generate an increase in viscosity of the medium and therefore the OTR would be adversely affected, causing a decrease in the grow rate [41]. This is especially critical considering that many high-density cultures end due to oxygen limitation [10], however, this could be solved by the flow of oxygen-enriched air [41]. In addition to the above, it should also be considered that continuous systems operated in cascade are more likely to become contaminated due to the multiple connections between each reactor; alternatively, the tubular flow piston reactors (CPFTR), can also be divided into several stages with different culture conditions, with the advantage that they allow to obtain higher product quality because the uniform physiological state of the cells can be kept, therefore they represent a promising and simpler alternative to operate [21].
C. necator production has also been reported growth-associated [24,35,40]. Alcaraz et al (2019) they evidenced that the formation of the polymer occurs from the growth stage due to an oxygen limitation in the culture, favoring the accumulation of PHA due to the increase of NADH in the cytoplasm, according to (Budde et al., 2010) [49], this accumulation inhibits the enzymes citrate synthase and isocitrate dehydrogenase, preventing acetyl-CoA from entering the tricarboxylic acid cycle. This causes the microorganism's metabolism to be directed towards alternative routes to maintain the NADH / NAD + redox balance, as is the case of the PHA formation route. And although in this route, the electron donor is NADPH, it has been reported that in some strains of C. necator, the enzyme acetoacetyl-CoA reductase involved in this route of synthesis of PHA, which uses NADPH as an electron donor in the reduction of acetoacetyl-CoA to 3-hydroxybutyryl-CoA, it is not NADPH-dependent and can use NADH to carry out this reduction [50,51]. So, it may induce an early polymer formation under low-oxygen levels, thereby favoring the diversion of acetyl-CoA towards the PHA synthesis pathway. The above not only indicates that the production of PHA by this bacterium can be regulated from the growth stage, but it would allow its production in continuous single-stage culture systems, eliminating the need for multiple stages or the retention of biomass and its recirculation, which would favor the culture by allowing a simpler control of the process. This shows that the production of this polymer with this strain can be regulated and directed either towards an early formation related to growth, or late by limitation of an essential nutrient such as nitrogen and phosphorus. Eventhough the above, better results of this species were observed in this review under conditions of nitrogen limitation, both continuous and fed-batch systems. So, based on the aforementioned and considering the accumulation characteristics of this species, productivity could be improved by using a cell-recycle continuous system inducing the production of PHA through nitrogen limitation, since cell recycle would allow obtaining cultures of high cellular density, added to the intrinsic advantages of the continuous systems. Additionally, the need to sterilize the substrate during the production stage could be eliminated, since the medium would be low in nitrogen, an essential nutrient that would limit the growth of unwanted microorganisms, which allows a greater economy in the process [14]. Besides, this strategy would solve one of the main disadvantages of the cultures of this species, which is the increase in viscosity in the medium due to the secretion of exopolysaccharides which limit OTR and, therefore, productivity, since by frequently eliminating the depleted medium, the increase of viscosity would be avoided [19].
4.5.2. Halomonas spp.
The first non-sterile open culture at pilot plant scale was reported by [47], operated in fed lots, and sterilization was not required throughout the process. In this study, tap water was additionally used instead of distilled water and a final productivity of 1.04 g/l.h was obtained. The non-sterile culture was used in all the articles that worked with the genus Halomonas, including the one that reported the highest productivity found with this species (1.59 g/l.h) [44], the authors used a mutant strain with an improved promoter of the orfZ gene that encodes 4HB-CoA transferase, to increase the production of P (3HB-co -4HB) in a fed-batch culture.
As for, Halomonas sp. TD01, was cultivated in a continuous system, with glucose as a substrate, and got a productivity of 1.52 g/l.h of PHB (Fig. 6), which is comparable to that of [44] despite that an isolated strain was used without genetic improvement (Table 2). This culture was carried out in two 6 L fermenters in open culture, pumping the culture from the first fermenter to a second nitrogen-deficient fermenter (two-phase process).
The authors highlight that the operating costs are reduced by using tap water for the culture medium instead of distilled water and by not needing sterilization due to the high concentrations of salt in the medium that inhibit possible non-halophilic contaminating organisms [45].
These non-sterile PHA production systems can address the contamination problem and have low operating costs, such as the open continuous system reported in [45]. In fact, this may be the next generation of Industrial biotechnology of PHAs production as mentioned by [52], because simultaneously it reduces energy consumption and drinking water consumption. In the same article, they highlight the implementation of a continuous system of 1000 L on an industrial scale with Halomonas bluephagenesis using filtered seawater. This would be a novel alternative that can significantly improve the process by using resources more efficiently. But there are also limitations associated with this type of system. Even moderate salinity eventually causes corrosion of stainless-steel bioreactors, which are the most widely used industrially [53].
4.5.3. Pseudomonas putida
This species has great metabolic versatility that allows it to adapt and thrive in various environments. They naturally produce mcl-PHAs, from various substrates such as glucose, glycerol, fatty acids, hydrocarbons and waste compounds [54]. The synthesis from fatty acids generates monomers related to them because it is carried out through the β-oxidation pathway, but when starting from carbohydrates, the synthesis always occurs through the acetyl-CoA precursor [18]. The route that leads to the formation of acetyl-CoA has the advantage that it allows using cheaper substrates than those required for β-oxidation such as sugars, simple waste glycerol and volatile fatty acids from fermented effluents. In addition, polymers with different levels of unsaturated monomers can be generated and functionalized to generate added value. It has even been reported that strain LS46 can generate scl-mcl hybrids since de novo synthesis and β-oxidation pathways can be active simultaneously [55]. This species was the only one found to synthesize mcl-PHAs in this review (Fig. 4).
The best productivity found in P. putida was 1.63 g/l.h in a fed-batch culture using phosphorus as a limitation (Table 2). Sodium butyrate was used as the initial carbon source and butyric and decanoic acids as feed substrates, and recorded a Yp/x of 0.65 g/g [43]. In another study conducted by [28], a productivity that reached 1.16 g/l.h was obtained in a growth-associated culture, using decanoic acid, acetic acid and concentrated glucose. In this strategy, acid acetic prevents decanoic acid from being used in the generation of energy and diverts it towards the synthesis of PHA, allowing the simultaneous formation of different monomer units and improves the efficiency of the process [28], obtained a Yp/x of 0.74 g/g, about 10% more compared to the previous study. However, a higher productivity was observed in the study of [43] which was performed not associated with growth, additionally, when fatty acids related to the type of PHA that will be produced are used [18], it is more profitable to have a stage of growth with simple substrates, such as sugars and then feed with fatty acids [31]. Consistently with the above, (Cagnone & Gil, 2017) [56] report that low phosphorus concentrations induce greater accumulation of PHAs, while the accumulation of PHAs and the production of cellular biomass presented little difference regardless of the nitrogen concentration. it is noted that the physiology of the microorganism conditions the growing strategy, as it should focus on optimally using this induction mechanism.
4.5.4. Azohydromonas lata
This species had the highest productivity reported in the review (4.20 g/l.h) [26], although only one article was within productivities ≥1.0 g/l.h. In this study, glucose was used as a substrate, without nutritional limitations (growth-associated production) in a fed-batch system and an Yp/x of 0.68 g/g was obtained. It is noteworthy that within the same study, another experiment was carried out with induction of PHA accumulation by nitrogen limitation, and although they obtained a higher Yp/x (0.94 g/g), the final productivity achieved was lower (1.8 g/l.h) than that reported under non-nitrogen limiting conditions.
This demonstrates that with this species, the production of PHA associated with growth, improves the productivity of the process even if the yield of biomass in product is lower [26]. This type of production would open up the possibility of being used in a continuous one-stage system [3], even so, in the specific case of A. lata, it is reported that a second stage could improve productivity due to longer residence time, which allows greater substrate conversion [21], therefore, this could be a way to improve production and the strategies mentioned above (such as using low-cost substrates) could be used to reduce process costs. As mentioned in the introduction, this constitutes the greatest obstacle to its economic feasibility, considering this (Surendran et al., 2020) [57] highlight that industrial wastewater can be used as a low-cost carbon source, and they focus in waste of edible oils of vegetable origin and in waste animal oils. In Fig. 5. it can be seen that with vegetable oils, fats were reported productivity greater than 1 g/l.h, specifically using coffee oil, waste vegetable fat and palm oil, using Cupriavidus necator strains in fed-batch systems (see Table 2) and compatible lipids such as monoacylglycerides, diacylglycerides, and free fatty acids produced during the decomposition of triacylglycerides from the substrate that can emulsify vegetable oil may be used to solve limitations such as low bioavailability of these substrates (due to their lack of homogeneity) and interference with OTR when accumulating on the surface [40].
Taking into account the aforementioned, knowing the limitations of each system and their kinetic characteristics of biopolymer formation is critical in defining the appropriate culture system for the chosen microorganism [58,59]. In fact, according to [60] the most critical aspect for the accumulation of PHAs is the chosen culture system, since, if the substrate concentration is too high, inhibition and loss of productivity can occur, and if administered by pulses (fed-batch) the limited volume and eventual consumption of the substrate will also be a limitation. For this reason, and because of what was observed in the articles on the different microorganisms on which this analysis was focused, it is generally recommended to use continuous or semi-continuous systems and microorganisms compatible with this culture system.
5. Conclusions
The continuous system was the least reported, however, the information found shows that for the 4 genera on which the analysis was based, productivity could be improved with continuous or semi-continuous systems (in the case of C. necator). According to the literature consulted, the characteristics of this system could increase productivity and iat the same time decrease the space required for the process; therefore, it is necessary to drive out more research in this regard
Nitrogen limitation can potentially improve the productivity of C. necator and H. bluephagenesis, while in the case of P. putida, phosphorus limitation reported the best results. In contrast, A. lata reported the highest productivity of PHAs without nutritional limitation, not only at the species level but also at the global level in the present review. This could indicate that the growth-associated production allows obtaining higher volumetric productivity since PHAs are synthesized throughout the process. In addition, the fact that the culture strategies varied in each species of microorganism indicates that the physiological characteristics of the microorganism together with variables such as the substrate used, the mode of operation and type of polymer determine the culture system.
The information found did not allow an integral comparison of all the mentioned variables for the same microorganism, but it did allow to make several general aspects in several species highlighted by their high productivity, therefore, it is evident that there is a lack of knowledge regarding the consideration of the joint effect of these variables in improving the bioprocesses of PHA production.
According to the results, the production of PHAs with C. necator strains can be regulated and directed towards an early formation related to growth, or late by limitation of an essential nutrient such as nitrogen and phosphorus, which determines the strategy that it would be used for its culture.
Regarding productivity, the maximum found was registered when employing sugars, a substrate that is generally not considered low cost. However, there were significant productivities associated with lipids such as vegetable and animal oils and fats or their constituents (fatty acids and glycerol). All of them industrial by-products that can reduce production costs.
Most of the publications focus on scl-PHAs (75%), however, mcl-PHAs and scl-mcl hybrids were little reported and only with two genera Pseudomonas putida and Cupriavidus necator respectively, and there is a great potential in terms of processing properties and added value to the final product and functionalization of these biopolymers that has not been sufficiently explored.