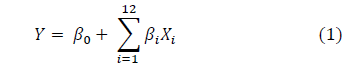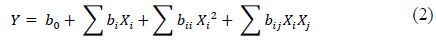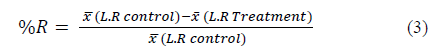1. Introduction
Livestock industry worldwide endures major economic losses resulting from diseases caused by parasitic agents. Charlier et al. 2009 reviewed the different effects caused by gastrointestinal nematode (GIN) infections in adult dairy cattle and reported a reduction on weight gain, less carcass quality, and a reduction in milk yield. All these factors are translated in reductions in farm profitability [1]. Some studies have tried to quantify this economic cost in different countries [2-5]. The cost of having parasitic diseases in cattle has been estimated in countries such as the United Kingdom, Switzerland and the United States. This cost, which includes losses in livestock productivity and chemical treatments, is worth approximately between €99 million to €400 million depending on the country [2]. Moreover, North American beef and dairy industries reported an annual cost of more than $2 billion in additional treatments with suboptimal productivity due to GIN infections [4]. Furthermore, in Latin America the annual economic loss in Mexico and Brazil due to GIN infections is worth $445.10 million and $13.96 billion, respectively [5].
The most common alternatives to control GIN infections are anthelmintic treatments, but these chemicals have limitations when they are misused. The use of chemical treatments in a prolonged manner, the employment of single-drug regimens, overdosed, and application without technical knowledge has caused the development of anthelmintic resistance in nematode populations [6,7]. However, new alternatives for the control of nematodes have been previously studied, including nematode-trapping fungi. These organisms are able to form trapping devices especially designed to trap nematodes, destroy them, and feed from them [8]. Among these, Duddingtonia flagrans stands out as a fungus with potential as a biological control agent, as it produces resistance structures known as chlamydospores. These structures are supplied to cattle by oral administration, and are capable of passaging through the gastrointestinal tract and resisting unfavorable conditions like ruminal fermentation, high osmotic pressures, high temperatures and microaerophilic conditions [9]. Finally, chlamydospores are excreted through the feces, and they colonize, persist and act over parasite larvae [10,11]. Therefore, researches worldwide have been focused in developing a bioproduct based on chlamydospores of D. flagrans, and specifically, to find a low-cost and competitive fermentation medium that generates high chlamydospores production.
Specialized authors in the subject established that chlamydospores production of D. flagrans can occur in liquid or in solid phases. Several researches have made progress in the study and optimization of chlamydospores production conditions, most of them employing submerged fermentation. According to Da Silva et al. 2015 the maximum chlamydospores productivity under solid state fermentation (SSF) reported 2.5x105 chlamydospores g dry substrate-1 day-1 using rice grits as a substrate for fermentation. Nonetheless, the productivity is still low and should be increased to have an economically profitable mass production process [12].
According to the aforementioned, the aim of this study was to increase production efficiency by evaluating the effect of different carbon and nitrogen sources, as well as sporulation inducers on a solid-state fermentation system for D. flagrans by using statistical optimization techniques. To the best of our knowledge, this paper is the first work where an optimization technique is applied to study the effect of different substances on the production of chlamydospores of D. flagrans.
2. Material and methods
2.1 Fungus
D.flangrans Agrosavia-BGMSABV-Df-Col-H-001-2014 strain is deposited at the RNC (for its Spanish acronym, Registro Nacional de colecciones biológicas) under the collection number 129-Banco de germoplasma de microorganimso-Corpoica. This fungus is a native Colombian strain which could be used under the Contract for Access to Genetic Resources and its Derived Products No. 168 de 2017.
The fungus was stored as discs of mycelium in agar suspended in cryopreservation solution at -20 ± 2°C at the Agrosavia working collection. For each experiment, the fungus was reactivated and cultured on wheat flour agar (WFA, 30 g L-1 of wheat flour and 18 g L-1 agar) at 28 ± 2°C for 7 days, and it was then subcultured for another seven days using the same media and under the same conditions. At this point, mycelium discs were used to prepare the inoculum for the SSF.
2.2 Inoculum preparation
Discs of mycelium were inoculated in 200 mL of modified Sabouraud medium (sucrose, 0.30 % wv-1 and peptone 0.10 % wv-1) in a 1,000 mL Erlenmeyer flasks. The flask was then incubated on a thermos-controlled rotatory shaker (LSI-1005P, Daihan, Labtech, Namyangju City, Korea) at 150 rpm and 28 ± 0.5 °C for 8 days.
2.3 D. flagrans chlamydospores production in solid state fermentation
Thirty-five grams of split rice were placed on aluminum trays (10 cm length x 8 cm width, and 5 cm height), moistened with 45 mL of water (pH 7.0 ± 0.5) and mixed thoroughly. Then, they were autoclaved for 40 minutes at 121°C. After sterilization, each tray was inoculated with 5 mL of inoculum containing 2x104 chlamydospores mL-1 under sterile conditions. Finally, the trays were placed on an incubation room at 25 ± 2°C for 14 days, without humidity control and without initial pH adjustment. At 3, 7 and 14 days of fermentation, three aluminum trays per day were taken out for destructive sampling. In each tray, colonized split rice subsamples from at least four random points were collected to form a sample.
2.4 Evaluation of nutritional effects
The Plackett-Burman design experiment (PBD) was used to identify the significant nutritional factors such as carbon and nitrogen sources and/or inducing substances affecting chlamydospores productivity of the D. flagrans Agrosavia strain. Solutions with carbon and nitrogen sources as well as with inducing substances were prepared separately and added to the medium before sterilization. The list of chosen substance (with concentration and code) is presented in Table 1.
Table 1 Definition of experimental variables for the evaluation of nutritional effects using the Plackett-Burman design strategy.
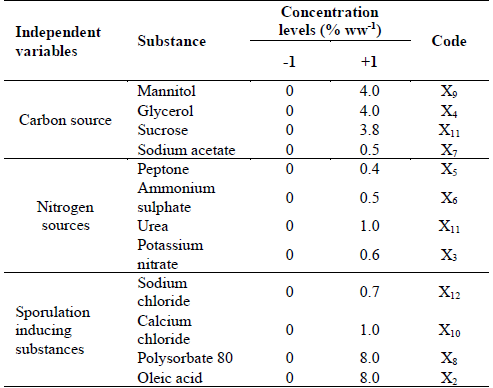
Source: The authors
Twenty experiments were carried out with 19 variables, where 12 of these were nutritional components (independent variables) and seven were dummy variables used to calculate the standard error. The general form of the linear regression model used is as follows eq. (1)
Where, Y is the response or dependent variable (chlamydospores per g-1 of dry substrate); β 0 is the model intercept; X i is the independent variable; and β i is the linear regression coefficient. The significance of each variable was determined by a one-way variance analysis (ANOVA). A value of p = 0.1 corresponds to a statistical confidence level of 90%, and therefore, any variable with a confidence p lower than 0.1 was considered significant. Since, the experiment is to evaluate the relative effect of each variable, a significance level of 90% is acceptable. The statistical analysis of the PBD was carried out using the software Minitab 17.0.
2.5 Response surface methodology (RSM)
An orthogonal 22 factorial central composite experimental design (CCD) with four-star points and eight replicates at the center, and with a total of 16 experiments was used to optimize the concentrations of effective nutrients, i.e. ammonium sulphate and sodium acetate, which resulted from the previous experiments (See Section 2.3.1.). The lowest and the highest concentrations of selected nutrients were tested at three levels, -1, 0, and +1.
The quadratic model for predicting the optimal point was expressed according to eq.(2):
Where Y is the response variable (chlamydospores g dry substrate-1), b are the regression coefficients, and X are the coded levels of the independent variables. The range and level of the variables are given in Table 2.
Table 2 Definition of experimental variables for optimization of important variables using a composite experimental design.
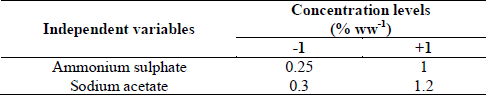
Source: Authors
An additional SSF experiment was conducted with optimal concentrations of ammonium sulphate and sodium acetate to verify optimal conditions. The experiment was run by triplicate and the results were reported as the average ± standard deviation
2.6 Determination of chlamydospores concentration
Five grams of rice colonized by D. flagrans Agrosavia strain were mixed with 50 mL of 0.01 % vv-1 Tween 80. The flask containing the mixture was stirred with a manual blender for 5 minutes to separate most of the chlamydospores from the substrate. Then, the number of chlamydospores was determined using a Neubauer counting chamber.
2.7 Determination of probability distribution of data
In order to establish the probability distribution of data (normal distribution) was performed the Anderson Darling statistic test, where was proposed two hypotheses for the Anderson Darling test (Ho= null hypotheses and H1 = alternative hypotheses)
A value of p = 0.05 corresponds to a statistical confidence level of 95%, and therefore, any variable with a confidence p lower than 0.05 was considered significant.
2.8 Evaluation of the predatory capacity of chlamydospores
Chlamydospores total count (1 x106) produced by SSF using split rice with/without the addition of ammonium sulphate and sodium acetate were cultivated in Petri dishes with water agar medium (Agar, 18 g L-1). These were incubated for 3 days at room temperature (22 ± 2°C). Then, two hundred Panagrellus redivivus infective larvae were placed on the surface of each dish and incubated for 2 days at room temperature. After this period, the surface of the Petri dishes was scraped with a spatula and keep in Baermann tubes for another 24 hours. Afterwards, the liquid was collected in assay tubes and the larvae recovered were counted with a microscope at 4X [13]. Eight replicates were also cultivated besides in water agar but without the fungus and used as control.
The reduction percentage in the mean number of larvae recovered was calculated employing eq. (3) [14]:
3. Results
3.1 Nutritional factors effects D. flagrans chlamydospores production
During the SSF process for D. flagrans chlamydospores production, significant variables were screened via the PBD.
From the literature review carried out, twelve nutritional components were identified as important for chlamydospores production by different fungi strains. Thus, a design matrix for the PBD strategy was created. Under these conditions, the highest concentration was achieved in Run 8 with 6.26x107 chlamydospores g dry substrate-1. Determination coefficient R2 was used to evaluate the fitness of the model established. In this model, the R2 obtained was 0.91. The effect of each factors was calculated by an analysis of variance (ANOVA) and it is depicted in Table 3.
Table 3 ANOVA for the Plackett-Burman design model for chlamydospores production. The underline variables have a positive significant effect.
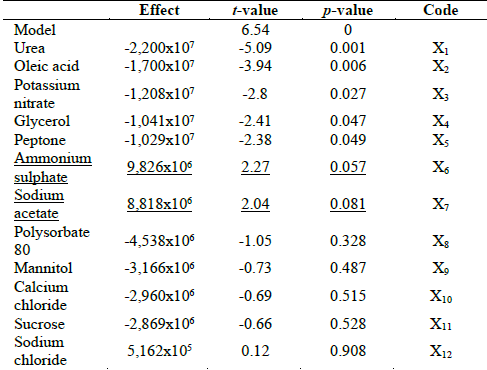
Source: The authors
Factors having a confidence level higher than 90% were considered to have a significant effect on the response and were further optimized. The lowest p-values indicate the most significant factors on chlamydospores production. From these experiments, the most important factors affecting, in a positive way, chlamydospores production were ammonium sulphate (p=0.057) and sodium acetate (p=0.081) (underlined in Table 4). Meanwhile glycerol (p=0.047), peptone (p=0.049), potassium nitrate (p=0.027), urea (p=0.001), and oleic acid (p=0.001) were significant but with a negative effect on chlamydospores production.
3.2 Optimization of selected nutritional variables
A CCD was used to figure out the optimal level of the two selected variables (ammonium sulphate and sodium acetate) to produce chlamydospores of D. flagrans. A total of 16 experiments were run. The coded variables and the levels of the variables, i.e. ammonium sulphate and sodium acetate, are depicted in Table 4.
Multiple regression analysis was applied to the CCD data. Also, transformed models such as square root, natural log and inverse square root were evaluated for the fittingness to the response outputs. The bests model generated was the inverse square root eq. (4), showing chlamydospores concentration as a function of ammonium sulphate and sodium acetate.
Where A is the ammonium sulphate concentration and B is the sodium acetate concentration. The ANOVA of the polynomial model is showed on Table 5 and demonstrates that it is significant at a 95 % confidence level base on a p<0.05. The R 2 value for the model was 0.9059, which indicated that 90.59% of the variations observed in chlamydospores production could be explained by the model.
Table 5 Analysis of variance for the fitted polynomial model of chlamydospores production of D. flagrans. A = Ammonium sulphate; B= Sodium acetate
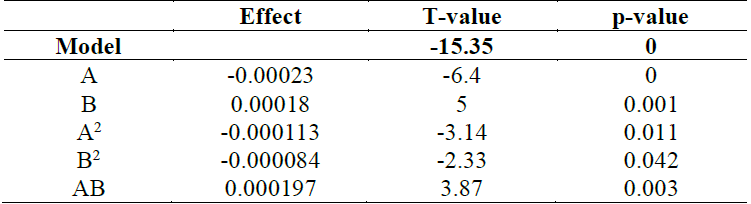
Source: The authors
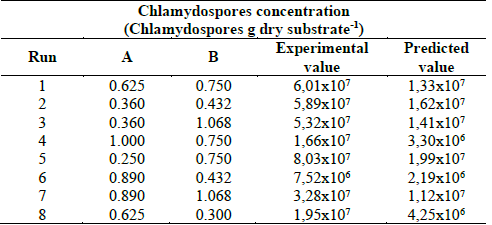
This result also suggested that the prediction of experimental data was satisfactory, and this was confirmed by the Fig. 1 were the experimental value and predicted value for chlamydospores production are presented. The R 2 value was of 0.951, which is a good fitting for this type of experiments where the variability between experiments is usually high, nonetheless, these kinds of variations are expected in experimental quadratic models.
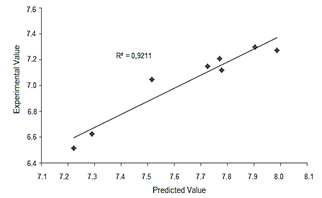
Source: The authors
Figure 1 Experimental value versus predicted value for chlamydospores concentration
To visualize much clearer the interaction of the two factors on chlamydospores activity, a graphical representation of response surface curve is showed in Fig. 2a. And the contour plot can be seen in Fig. 2b. The response surface obtained in this study was convex in nature. This suggested that the optimum conditions were well defined under the range studied. Fig. 2 indicated that the interaction between the ammonium sulphate and the sodium acetate was significant, and is supported by a p=0.003 obtained in the ANOVA (Table 5). Also, the curve of Fig. 2a is due to the significant effect of the quadratic coefficient of ammonium sulphate (p=0.011) and sodium acetate (p=0.042). Furthermore, extremely high values of only one of these variables will inhibit chlamydospores production (Fig. 2b), and on the contrary, the optimal region for chlamydospores production is between 0.25-0.50% of ammonium sulphate and 0.40-0.95% of sodium acetate.
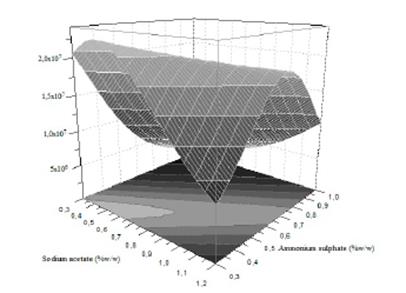
Source: The authors
Figure 2a Response surface curve plot of the interaction effect between ammonium sulphate (% ww-1) and sodium acetate (% ww-1) on chlamydospores concentration (chlamydospores g dry substrate-1).
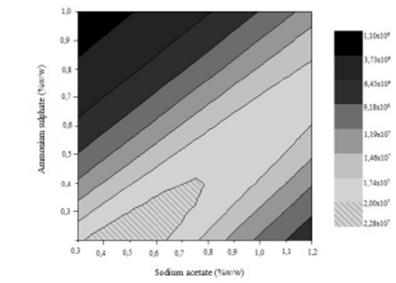
Figure 2b Contour plot of the interaction effect between ammonium sulphate (% ww-1) and sodium acetate (% ww-1) on chlamydospores concentration (chlamydospores g dry substrate-1).
The adequacy of the predicted model (Equation 4) was confirmed by carrying out eight additional and independent experiments employing the suggested optimal conditions and the results are showed in Table 5. The experiments were performed in trays using split rice combined with 0.250 % ww-1 of ammonium sulphate and 0.5636 % ww-1 of sodium acetate. Chlamydospores concentration of 2.71x107 Chlamydospores g dry substrate-1 with a coefficient of variation of 6.49% was obtained and was found to be higher than the predicted concentration of 2.26x107 chlamydospores g dry substrate-1 at 14 days of fermentation.
3.3 Determination of probability distribution
With the Anderson Darling test, it was determined that the data presented a normal distribution. The data obtained from the evaluation of nutritional conditions presented a p value of 0.560 and the data obtained for the optimization of the selected nutritional variables presented a p value of 0.307. In both cases, the p value obtained from the Anderson Darling statistic test is greater than 0.05, therefore the null hypothesis is accepted, establishing that the data follows the normal distribution.
3.4 Evaluation of the in vitro trapping capability of nematophagous fungi against Panagrellus redivivus
The P.redivuss nematode population (third stage larvae) treated with the chlamydospores obtained from the fermentation process using rice with the addition of ammonium sulphate and sodium acetate as substrates, showed a significant reduction of 12% in the mortality, compared with the treatment of rice without ammonium sulphate and sodium acetate, as confirmed by the Tukey test (data not shown). Predatory capacity results showed an average dead nematodes value of 84 ± 5% in the treatment with the addition of ammonium sulphate and sodium acetate, meanwhile in the treatment without ammonium sulphate and sodium acetate the mortality was 96.2 ± 1%.
4. Discussion
Higher productivity of resistance structures of nematode-trapping fungi is an important factor for biological control and its production at an industrial scale. Chlamydospores are formed from asexual reproduction processes and are specific to each microorganism; however, there are nutritional conditions, changes in osmolarity, light, pH, induction substances, and temperature that can promote the formation of these structures [15]. Some of the substances that have already been studied are glycerol, mannitol, sucrose, urea, polysorbate 80 (Tween 80), biotin and organic or inorganic nitrogen sources. These studies evaluated different species of nematode-trapping fungi such as Arthrobotrys oligospora, Monacrosporium cytosporum, Arthrobotrys conoides, Drechslerella stenobrocha and D. flagrans, as well as other fungi that do not have nematode trapping activity, but which have been well studied for the production of chlamydospores such as Giberella zeae, Pochonia sp. and Candida albicans [16-20].
In this study, four substances were tested as a nitrogen source, i.e. ammonium sulphate, peptone, urea and potassium nitrate. From these, the last three cause a decrease in productivity, meanwhile, ammonium sulphate has a positive effect on chlamydospores production of D. flagrans Agrosavia strain. This behavior could be explained through the mineralization-immobilization-turnover (MIT) route that has been studied in different fungi and bacteria [21]. When complex nitrogen molecules such as urea and peptone are present, the microorganism must produce extracellular depolymerases (ureases, proteases, among others) for the hydrolysis of these compounds, causing an additional energy cost. On the contrary, when ammonium sulphate is available, the ammonium ions are transported directly by membrane proteins, reducing the energy consumption and repressing the utilization of alternative N sources such as nitrate (NO3 -) and organic molecules [21].
The addition of glycerol to the fermentation substrate had a negative effect on the production of chlamydospores. Nonetheless, it has been reported that polyols such as glycerol and mannitol could be used as a reserve source when the cell is facing adverse conditions. Additionally, they are important antioxidant substances used as osmotic control in cells [18]. In this case, D. flagrans could not use this compound (causing a negative effect) since there was no water stress, because the relative humidity of the substrate used in the fermentation process at 14 days was 55 ± 5 %.
On the other hand, sodium acetate showed a positive effect on chlamydospores production. Gardner et al. [16] evaluated the addition of this compound at concentrations of 1 % wv-1 obtaining a productivity of 4.64x103 chlamydospores mL-1 day-1. Several authors demonstrated that small chain carboxylic acids such as acetate are converted to acetyl CoA via the citric acid pathway, and previous studies have also reported that small amounts of short chain acetate and carboxylic acid could stimulate the production of zygospores and chlamydospores [22]. Finally, [23] showed that in Phycomyces sp. and Blakeslea sp. (spore-producing fungi) these developed more abundant zygospores and density in a medium with acetate at 0.08 % wv-1.
The highest chlamydospores productivity (1.62x106 chlamydospores g dry substrate-1 day-1) for D. flagrans employing SSF had been achieved in this study, compared with previous works. Sagues et al. [19] concluded that the medium must be enriched with nutrients needed for growth and sporulation of D. flagrans, but the maximum productivity reported was 7.18x106 chlamydospores mL-1 day-1. Moreover, Santurio et al. [24] reports the production of chlamydospores using rice without the addition of inducing substances or any additional carbon or nitrogen source with a value of 5x104 chlamydospores g dry substrate-1 day-1. Castillo Saldarriaga et al. [25] achieved a productivity of 7x105 chlamydospores g dry substrate-1 day-1 using split rice as substrate. Finally, Silva et al. 6 attained a productivity of 2.5x105 chlamydospores g-1 dry substrate day-1 using rice grits as a fermentation substrate.
In this study, D. flagrans showed a predatory capacity of 96.2 ± 3% from chlamydospores obtained from an SSF process without the addition of substances. However, the chlamydospores obtained from the SSF process with addition of ammonium sulfate and sodium acetate showed a 12% reduction in its predatory capacity. Thus, an inhibitory effect of the substances was considered. Previous reports have studied the effect of different carbon and nitrogen sources to the trap formation of D. flagrans F_882, they found that ammonium ions (0.2-0.4%) increased the number of traps, nonetheless higher ammonium ions concentration (0.8%) decreased the production of traps [26]. To confirm the inhibitory effects of ammonium ions over the predatory capacity of D. flagrans Agrosavia strain some additional experiments were done reducing the concentration of ammonium ions (0%-0,3%), but keeping the sodium acetate concentration at 0.5636 % ww-1. The predatory capacity decreased as the ammonium ions concentrations increased, a loss of 5.6% of predatory capacity is reached when ammonium ions are at 0.3%. Contrary, the productivity increased as the ammonium ions increased, at 0.3% of ammonium ions the productivity increased 5.8 times (1.27x106 chlamydospores g dry substrate-1 day-1) compared to the productivity when no ammonium ions are presented in the media. Thus, ammonium ions induced chlamydospores production, but affect the predatory capacity. We hypothesized that this behavior is due to the chlamydospores during the SSF are saving ions ammonium in its structure. This behavior is delaying the switch of the chlamydospores to saprophytic mode when its growth in media (water agar) used to evaluate the predatory capacity in vitro cultures [27]. Nutrional and genetic engineering studies [28]. 7report that a prerequisite for significant trap formation is when the fungi is grown on a low nutrient mineral salts medium. This might indicate that nutrient limitation favors induction of trap formation. Nonetheless, even when the chlamydospores are grown in water agar and the nematodes become an important source of nitrogen during growth of the fungi, the chlamydospores produced from SSF with ions ammonium do not require them.
Predatory capacity results are consistent with previous studies that show that the predatory capacity of D. flagrans against larvae of C.elegans, H. contortus and P. redivivus was 93%, 99.5% and 83%, respectively [29]. Gonzalez et al. [30] studied the predatory capacity of D. flagrans in three different media, water agar: 90 ± 2.1%, corn meal agar (CMA) 89.9 ± 1.4%, and potato dextrose agar (PDA) 74 ± 1.9%, finding significant differences (p <0.005) and concluded that the addition of substances has a negative effect at predatory capacity.
Using chlamydospore as a biological control of parasites in cattle does not represent a risk over the health of cattle due to these structures have washing processes to eliminate possible extracellular substances or metabolites excreted in the fermentation medium, also secondary metabolites from trapping fungi as an oligosporol, hidroxyoligosporon, flagranone, arthrobotrisin are metabolites that have not been studied extensively and the studies reported demonstrate only nematicidal effect.
5. Conclusions
The results obtained in this work show the highest productivity value of 1.62x106 chlamydospores g dry substrate-1 day-1 with the addition of inducing substances and employing SSF. Despite the addition of the substances evaluated, there is an increase in productivity of 15% compared to the treatment that does not have ammonium sulphate and sodium acetate, causing a reduction of 12% in the predatory capacity of D. flagrans