1. Introduction
The valuation of a mineral deposit often involves only a generic study of the grades of the interesting elements that exist in a type of deposit, but a detailed study of the mineralogy can help to increase the added value of the mining operation [1].
Mineralogical characterization is a fundamental step in designing and implementing metallurgical extraction processes and mine planning in general. In the case of gold mining, knowing how gold occurs, as well as its granulometry and mineralogical associations, is a fundamental tool when minimizing losses due to inadequate recoveries, or recognizing and designing processes, taking into account the presence of minerals that are detrimental to the different extraction processes [2,3].
The comprehensive determination of mineralogy, mineral associations, and gold deportment is increasingly being recognized as an integral part of any gold evaluation project. Whether looking at pre-feasibility, feasibility, or process optimization, several studies have demonstrated that a greater understanding of sample mineralogy along with information on gold deportment can enhance the applicability of the results [4].
Historically, gold deportment characterization has been used extensively in process definition and optimization. The characterization of gold in a deposit is very important, as this helps determine exploitation and metallurgical extraction processes. Native gold (Au) and electrum (Au, Ag), found in various types of gold deposits, are the two most common and most important gold minerals. According to the mode of occurrences, gold can be classified into three categories: microscopic gold, submicroscopic gold, and surface-bound gold [4,5].
Native gold usually contains low concentrations of silver and, in some cases, mercury, copper, and palladium. It may also occur as inclusions within sulfur-rich minerals, such as pyrite and arsenopyrite. The style and variety of gold mineralization are influenced by the geological setting, chemistry of the ore fluids, and the nature of their interactions with rocks. [6].
Similarly, gold ores can be classified as “free-milling” and “refractory” depending on their response to cyanide leaching. While high gold recoveries (>90%) from free-milling ores can be readily achieved, refractory gold ores are often characterized by low gold extractions (50% - 80%) within a conventional cyanide leaching. The refractoriness of gold ores can result primarily from the inherent mineralogical features with particular reference to the mode of presence and association of gold, and to the presence of carbonaceous matter [7]. Processing inefficiency is caused by not implementing gold ore evaluation and characterization [8].
There are 17 mining towns and more than 30,000 artisanal gold miners in northeast Antioquia. Social and security issues in rural areas of Antioquia pushed miners to bring their gold ores to the towns near the mines to be processed in processing centers, or “entables” in Spanish. These centers operate in the urban areas amalgamating whole ore, i.e., without previous concentration, and later burn gold amalgam without any filtering/condensing system. Based on the mercury mass balance in 15 processing centers, or “entables,” 50% of the mercury-added to small ball mills, known as “cocos” in Spanish, is lost: 46% with tailings and 4% when amalgam is burned. Based on the above balances and Colombia's gold production, this region is the most contaminated in the world per capita from artisanal mining, with an average of 92 (73 - 110) tons of mercury per year [9,10].
According to data from the National Administrative Department of Statistics (DANE), in 2019, Colombian gold exports in March grew 33.8% over the same period in 2018, with transactions reaching US $155.6 million [11]. In the first half of 2018 alone, Colombia exported 532,372 Troy ounces of gold worth over US $642 million, with Antioquia being the department with the highest gold production in the country, contributing over 60% of the national production [12].
In municipalities in northeastern Antioquia that exploit gold, such as Segovia, Remedios, Anorí, Amalfi, and Vegachi, artisanal mining has a significant presence, along with its parallel problems of illegal exploitation, indiscriminate use of mercury and cyanide, informal gold extraction, mining accidents, and river contamination. Two alternatives for solving the problem of mercury in gold mining have been proposed [13]: mining legalization, which is the government’s responsibility from a social policy standpoint [14,15,16], and improvement of mining processes [17-19].
The main objective of this study was to perform a mining and metallurgical characterization of several gold deposits located in a region of the country with the highest gold production and complicated access due to social problems [20]. This characterization was performed using X-ray diffraction, X-ray fluorescence, QEMSCAN ®, SEM-EDX, fire assay, conventional cyanidation, and froth flotation.
2. Materials and methods
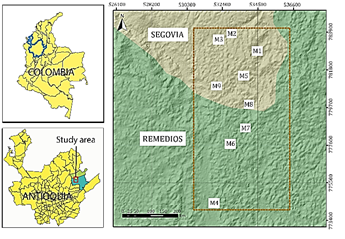
The northeastern part of Antioquia has distinguished itself over the years as one of Colombia's primary gold producing areas. In 2018, the gold production (in kg) in the municipalities in the region was as follows: Yolombó 17, Santo Domingo 20, Yali 33, Anori 62, San Roque 91, Vegachí 94, Amalfi 375, Remedies 3,546; and Segovia 4,553 [21]. More than 80% of gold production is concentrated in the municipalities of Segovia and Remedios.
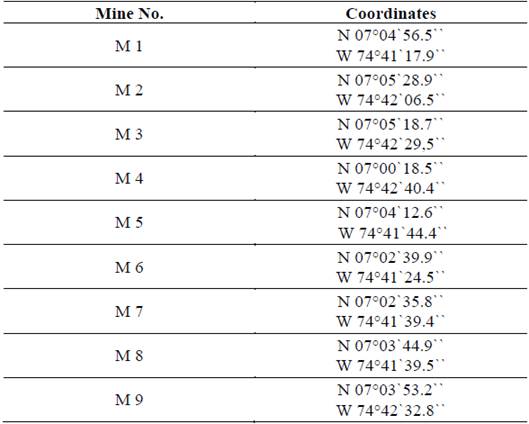
The samples collected for the study come from 9 mines located between the two municipalities with the highest gold production in the region, as shown in Fig. 1, and which are currently benefiting from the use of mercury. Each sample’s name and location is shown in Table 1.
From each of the chosen mines, approximately 30 kilograms of sample were collected. This ore mass was crushed and ground with the equipment that each mine has for this purpose. From the 30 kilograms, a quarrying process was carried out until a sample of approximately 10 kilograms was obtained, representative of each of the deposit’s exploitation fronts. The mass needed for each characterization test was obtained from each location’s 10-kilogram sample.
Sample characterization consisted in the application of the following techniques:
1) X-Ray Diffraction (XRD): To determine the phases or minerals present in each deposit, PANalytical XPert PRO MPD reference equipment was used in 2θ intervals between 4° and 70°, with a passage of 0.02° and an accumulation time of 56 seconds. A copper anode was used with Kα = 1.5406 Å.
2) X-ray fluorescence (XRF): PANalytical reference equipment and the AXIOS model determined the chemical elements present in the nine mineral samples, using a quantitative analysis of beads by dispersive X-ray fluorescence wavelengths.
3) The quantitative evaluation of minerals was carried out by scanning electron microscopy (QEMSCAN) [22].
4) Scanning electron microscopy with scattered X-ray energy (SEM-EDX): this technique was used to determine particle morphology and point chemical identification, through a sample analysis mounted in bakelite and specular polishing. This analysis was performed in a scanning tunneling microscope, reference Phenom XL.
5) Fire array and atomic absorption spectrophotometry (EF-EAA): This test was performed according to ISO 10378:2016 [23].
6) Conventional cyanidation tests: these leaching tests were conducted in a 2-liter stirred reactor. The pulverized ore was suited in a pulp of 30% solids, and the pH of the pulp was kept at 10.5 (with calcium hydroxide). The cyanide concentration was 0.04 M, and the leaching time was 48 hours. The leach liquors were analyzed in a Thermo Scientific iCE 3300 reference atomic absorption spectrophotometer to determine the amount of gold leached.
7) Froth flotation tests: these tests were carried out with DENVER D-1 reference flotation equipment. The pulverized mineral was suited in a pulp of 30% solids in a 5 liter cell. The temperature of the pulp was kept at 25°C. The collector conditioning time, flotation time, and agitation speed were kept constant at 5 min, 70s, and 118rpm, respectively. The rougher concentrate and process tails were analyzed by fire assay to determine gold recovery.
3. Results and discussions
3.1 XRD analysis
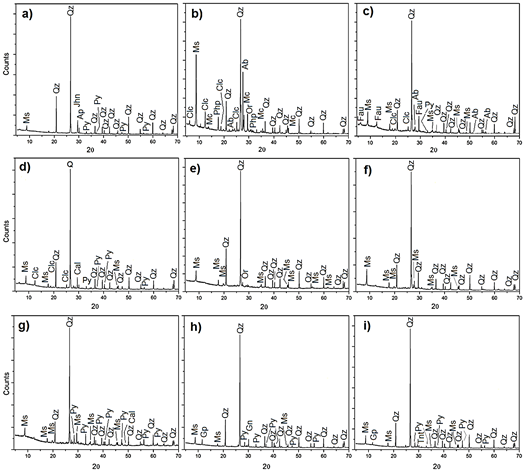
The XRD analysis of the nine points determined the mineral phases present in the samples, as shown in the diffractograms in Fig. 2, where most minerals in all points are quartz, muscovite, and pyrite. There are also minerals of minor importance, such as apatite, microcline, clinochlore, albite, and orthoclase, among others.
Source: The Authors.
Figure 2 Powder diffraction patterns, a) M 1, b) M 2, c) M 3, d) M 4, e) M 5, f) M 6, g) M 7, h) M 8, i) M 9. Legend [24]: Ms = muscovite; Qz = quartz; Py = pyrite; Ap = apatite; Jhn = johannsenite, Clc = clinochlore; Mc = microcline; Php = phillipsite; Ab = albite; Or = orthoclase, Fau = faujasite; Cal = calcite, Gn = galena; Gp = gypsum, Tnt = tennantite.
3.2 FRX analysis
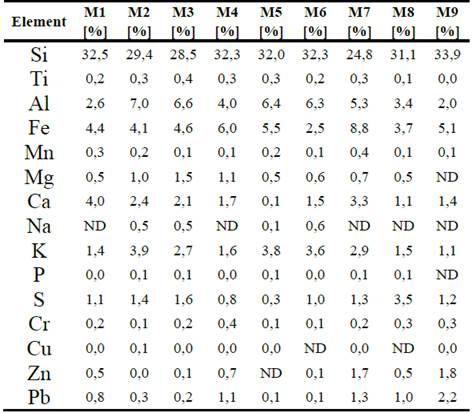
From the results obtained using XRF, the elements that make up the majority of the nine points analyzed is corroborated. Table 2 shows that the most common chemical element is silicon. The presence of other elements is also determined, such as aluminum and iron and, to a lesser extent, sulfur and potassium.
3.3 QEMSCAN analysis
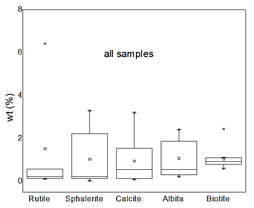
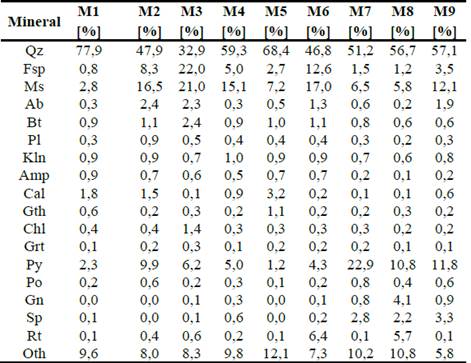
Through the analysis by QEMSCAN®, the percentage composition of the minerals present in the studied deposits is given in Table 3. All samples are composed of quartz, between 77% and 46% in weight, followed by muscovite (between 2% and 17%), pyrite (between 1% and 22%), and feldspar (between 0.8% and 22%), as shown in Fig. 3. Other minerals with minor presence are rutile (between 0.1% and 6.4%), sphalerite (between 0.01% and 2.7%), calcite (between 0.1% and 3.2%), albite (between 0.21% and 2.2%), and biotite (between 0.5% and 2.4%), as shown in Fig. 4.
Table 3 Mineralogical composition of gold deposits. Legend [24]: Qz = quartz; Fsp = feldespar; Ms = muscovite; Ab = albite, Bt = biotite; Pl = ;Plagioclase Kln = kaolinite; Amp = amphibolite ; Cal = calcite, Gth = goethite ; Chl = chlorite, Grt = garnet; Gy = pyrite, Po = pirrotine, Gn = Galena, Sp = sphalerite, Rt = rutile, Oth = others.
Source: The authors
Source: The authors
Figure 3 Box plot QEMSCAN® mineralogy for principal components for all samples, as a percent of a crystalline fraction. The median is the black horizontal bar; the box is the central 50% of simple frequency. Whisker ends are minima and maxima.
3.4 SEM-EDX analysis
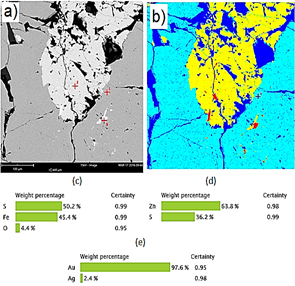
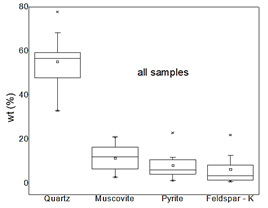
The shape and morphology studies were done using SEM images, and the elemental analysis of the various mines was done using SEM-EDX. Only some representative results are reported, which are for M2, M3, M7 and M9 samples. Fig. 5(a) shows the M2 sample SEM micrograph, with four points chosen for EDX analysis. Based on the grayscale, there is a matrix composed of three phases: iron sulfide (Fig. 5b), zinc sulfide (Fig. 5e), and silica (Fig. 5d) with the presence of metallic lead (Fig. 5c).
Source: The authors
Figure 5 (a) SEM image of a polished section of sample M2. (b) Chemical composition of point 1 iron sulfide. (c) Chemical composition of point 2 metallic lead. (d) Chemical composition of point 3 silica (e) Chemical composition of point 4 zinc sulfide.
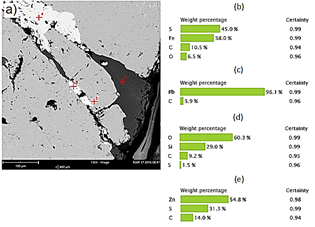
Fig. 6(a) corresponds to the image obtained by SEM for sample M3. Four points were analyzed for EDX analysis. Based on the chemical analysis in this mineral sample, the matrix is composed of zinc sulfide (Fig. 6b, and 6d), silica (Fig. 6c), and iron sulfide (Fig. 6e). In this sample, the presence of fluorine, bromine, and sodium is also detected.
Source: The authors
Figure 6 (a) SEM image of a polished section of M3 ore. (b) Chemical composition of point 1 zinc sulfide. (b) Chemical composition of point 2 silica (c) Chemical composition of point 3 zinc sulfide. (e) Chemical composition of point 4 iron sulfide.
Fig. 7(a) shows an SEM image of the M7 sample, and the only point observed is reported by the EDX analysis as electrum (a gold-silver alloy). A false-color image is shown in Fig. 7(b), where it is determined that there is gold (white color) immersed in an iron sulfide (green color) and calcite (blue color) matrix
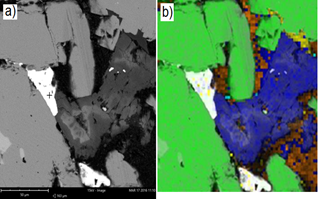
Source: The authors
Figure 7 (a) SEM image of a polished section of sample GD 7. (b) False-color composition; green: iron sulfide; blue: calcite; white: electrum.
Fig. 8(a) shows an SEM image of the M9 sample plus three points analyzed by EDX. A false-color image is shown in Fig. 8(b), where the light blue phase is iron sulfide, the yellow sector is zinc sulfide, and the red zone is, again, a gold-silver alloy (electrum). Based on the color designation, there is gold (Fig. 8e) immersed in a matrix composed of two sulfides: iron (Fig. 8c), and zinc (Fig. 8d).
3.5 Determination of gold by the fire assay method
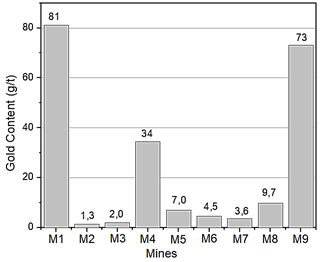
The amount of gold present in each mineral sample was determined by the conventional fire assay method, in triplicate. The results of this assay are shown in Fig. 9, where it is proven that gold mines in northeastern Antioquia currently belong to high tenor deposits, since an average of 24 g/ton of gold is calculated for the nine locations analyzed
3.6 Cyanidation and flotation tests
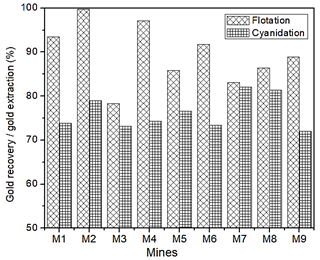
Cyanidation and flotation tests were performed to determine gold recovered under normal cyanidation or conventional flotation tests. Fig. 10 shows that by pre-concentrating the gold ore by a flotation process and then treating it with cyanidation, a greater quantity of gold is able to be recovered than with the conventional cyanidation process. With the pre-flotation process, an average of 90% gold extraction was calculated, with a minimum of 78% and a maximum of virtually 100%. In contrast, with the conventional cyanidation process, an average of 76% is calculated, with a minimum of 72% and a maximum of 82% gold recovery.
4. Conclusions
In general, gold deposits found in northeastern Antioquia have the following characteristics:
The analyses classify these deposits as high grade, since high concentrations of gold were determined in three of the nine deposits, with average values of 34, 73, and 81 g/t of gold.
Quartz is the most common mineral in the nine samples analyzed, with an average of 57%, followed by the presence of minerals such as muscovite (11%), pyrite (7%), and potassium feldspar (4.5%). The minority minerals are biotite with 1%, albite and calcite with 0.7%, and sphalerite and rutile with 0.2%.
Gold was detected in the form of electrum associated with sulfide minerals such as pyrite and sphalerite. In order to recover the precious metal, a conventional flotation process prior to leaching was proposed, since an average gold recovery of 90% was achieved for all mineral samples with respect to the conventional cyanidation process, where an average gold recovery of 76% was achieved.