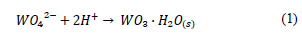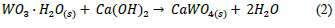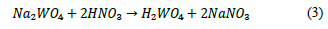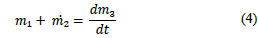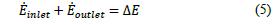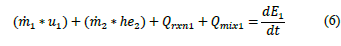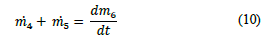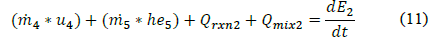1. Introduction
Tungsten is a specialty metal bearing unique hardness properties. Its alloys and products have prompted the development of tools and other technological materials that are widely used in industry, particularly in the aerospace field [1]. However, the tungsten ores availability has significantly decreased over the last decade, promoting the use of secondary sources such as tungsten carbide scraps, as an immediate alternative to fulfill the global demand for tungsten powder and other derivatives [2-5]. Thus, urgent efforts to explore novel secondary tungsten sources are required to mitigate the production shortage.
More recently, tungsten has attained significant importance in electroless nickel (Ni-P-W) coating processes, considering its co-deposition provides exceptional mechanical and chemical resistance to all types of treated surfaces [6,7]. Such properties are of the utmost importance in the oil-gas industry, where the durability of drilling tools, that are constantly exposed to erosive and corrosive environments, is a crucial factor in the operation costs [8]. As consequence, the Colombian oil-gas industry has been incorporating the nickel coating in their drilling and secondary oil recovery operations, where Field Service Solution (FES) S. A. S. is contributing significantly as a leader company in the design and implementation of this cutting-edge technology.
The typical composition of an electroless nickel (EN) bath includes sodium tungstate (Na2WO4) in concentrations as high as 25 g/L along with other additives such as sodium hypophosphite, the reducing agent, and organic carboxylic acids acting as nickel ligands and pH stabilizers [9]. The elevated tungsten concentration and its low co-deposition ratio make these types of solutions a suitable source of tungsten for secondary recovery operations.
It should be noted that only nickel recovery strategies have been implemented in spent EN baths [10]. Moreover, after an extensive review in scientific and technical literature, no tungsten-related processes have been explored for this type of industrial waste, whose enormous global generation rates boost the urgency to investigate supplementary recycling treatments.
Tungsten recovery from tungstate ion solutions has been widely discussed in literature. Strategies involving the use of ion exchange resins [11] and the selective precipitation and coagulation with complexing agents [12] and inorganic acids [13] have successfully allowed tungsten separation. However, in the case of EN solutions, the high concentration of other additives and ions may interfere with both the resin exchange process and the organic ligands complexation. Thus, acid precipitation constitutes a viable, selective, and low-cost alternative for the tungsten recovery treatment of EN waste solutions.
In this contribution, the design considerations of a pilot plant for scheelite production, directly from spent EN solutions, is described; this, based on laboratory-scale experimentation. This process will be implemented as a tungsten recycling alternative in the FES S. A. S. electroless nickel plant, located in Floridablanca, Santander, Colombia, to provide a greener character to the vanguard nickel coating process developed by our company.
2. Materials and methods
The electroless nickel solutions were acquired from Dr. Hesse GmbH & Cie KG, and were employed for tungsten recovery after depletion of the nickel content in the electroless nickel plant of FES S. A. S. (Floridablanca, Santander, Colombia). The chemical composition of the EN solution was not disclosed by the manufacturer. For the acid-precipitation experiments, hydrochloric acid (37 %, Merck KGaA), nitric acid (65 %, Merck KGaA), calcium hydroxide (>95 %, PanReac AppliChem) and commercial nitric acid (53 %, unspecified brand), were employed.
The tungsten concentration in different batches of spent EN solution was monitored by atomic absorption (AA) spectroscopy (Agilent 240 Series AA spectrometer operating with a nitrous oxide-acetylene flame). Powder XRD experiments were performed on a D8 focus Bruker diffractometer operating in a Bragg-Brentano geometry and equipped with an X-ray Cu tube (40 kV and 40 mA) and a mono-dimensional Lynx Eye detector. The quantitative crystal phases analysis was carried out employing the Rietveld method using aluminum oxide (corundum α-phase) as internal standard. The elemental composition was established with a SEM-EDS JEOL model JCM-6000PLUS microscope using an acquisition voltage of 15 kV, a probe current of 7.5 nA and ZAF correction.
3. Description and optimization of the process at laboratory scale
Preliminary characterization by AA of the spent EN solutions allowed to measure the tungsten concentration range between 6300-14000 mg/L. However, the high variability found among the different spent solution batches is attributed to the operational conditions of the autocatalytic nickel process in plant, the type and thickness of the applied Ni-coating, and the evaporation processes during storage, considering open containers are employed.
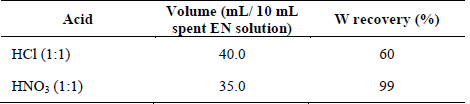
The experiments at the laboratory scale were carried out using 10 mL of a 7500 mg/L tungsten spent EN solution. The tungstite precipitation (WO3·H2O) (eq. (1)) was initially investigated by the addition of analytical grade hydrochloric and nitric acids in a 1:1 (v/v) concentration, at room temperature. The acid dosages found to provide the maximum tungsten recovery under these conditions are described in Table 1. The solid formed was easily recovered by filtration and dried at 90° C for 12 hours in all cases.
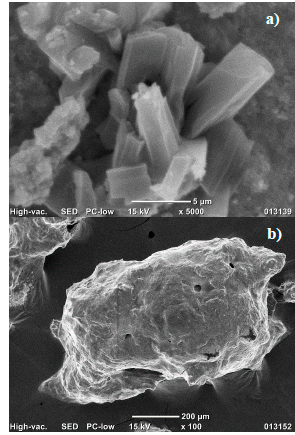
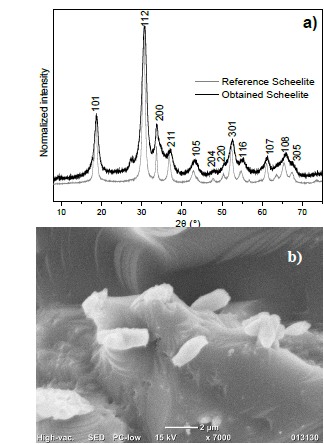
SEM-EDS and XRD characterization confirmed that the yellow solid product obtained after the addition of HCl corresponds to tungstite with a purity greater than 99 % and good crystallinity (Fig. 1). Nevertheless, despite an outstanding tungsten recovery was achieved when HNO3 is employed, the isolated white product is made of tungsten oxides and phosphite/orthophosphate salts amorphous agglomerates (Fig. 1) [14].
The formation of the previously mentioned mixture of tungsten oxides and the simultaneous oxidation of the hypophosphite ions, initially found in the spent EN solution, can be explained in terms of the weaker acidity and stronger oxidizing character of the HNO3, compared to HCl [15].
Once the addition of HNO3 was established as the best experimental procedure in terms of tungsten recovery, the amorphous solid was brought into reaction with a Ca(OH)2 0.1 M solution at room temperature (eq. (2)). The insoluble scheelite (calcium tungstate, CaWO4) formed was immediately recovered by filtration with a 73 % purity.
Considering that the combination of tungsten oxides and calcium hydroxide is an acid-base type reaction, the rapid scheelite formation leads to a decrease in the size of the crystals, as corroborated by the peak broadening observed in the XRD pattern, when compared to reference scheelite and to the SEM micrograph shown in Fig. 2.
Because of an operational cost optimization in the scaling-up of the process, the effect of non-analytical grade nitric acid (50 % w/w) was explored. Interestingly, a 1:1.2 ratio (spent EN solution volume: HNO3 volume) achieved an equivalent tungsten recovery percentage compared to the analytical-grade reagent. Moreover, only a 6 % difference in the final product content of scheelite was observed when comparing the crystal phases composition (Table 2).
Table 2 XRD characterization of the final products obtained with different types of nitric acid
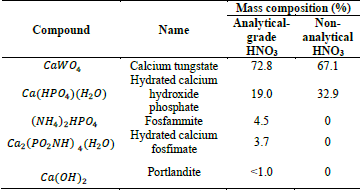
Source: authors
These outcomes indicate that the type of the nitric acids evaluated has no major effect in the final product quality. Hence, the non-analytical grade nitric acid was selected as raw material for the design of the tungsten recovery plant.
4. Pilot plant design considerations
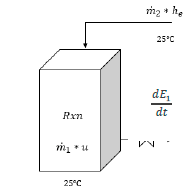
Based on the laboratory scale results and the chemical reactions involved, a general scheme for the pilot plant is presented in Fig. 3. The process starts with the reaction of tungstate ions in the spent EN solution with nitric acid (50 % w/w) in a 1:2.2 volume ratio in the reactor R-101, yielding an insoluble solid, whose composition has been previously discussed. Since the addition of an inorganic acid is taking place, we propose to maintain the temperature inside the reactor below 30° C employing an aerator-cooler to guarantee product conversion, avoid the generation of by-products and to maintain safety operative conditions. A stirrer is also contemplated to facilitate reactivity.
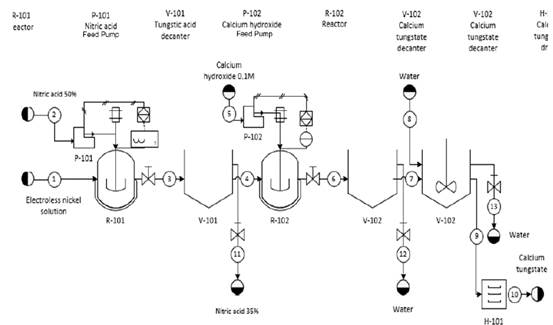
Source: authors Note: The decanter V-102 is presented twice to enlarge design details
Figure 3 Developed process flowsheet
The insoluble material obtained (mixture of tungsten oxides) is separated in the decanter V-101, where the supernatant unreacted solution is removed and the solid is then transferred to reactor R-102. Next, the Ca(OH)2 0.1 M solution is added until a pH of 7.0 is reached. Again, the temperature is kept under 30° C and a stirrer is projected to enhance reactivity. At this point, the scheelite is formed and is subsequently separated in the decanter V-102. Once the supernatant is removed, a wash cycle with distilled water is carried out to eliminate soluble impurities. Then, the solid material is separated again in the V-102 unit and, placed in the drying oven H-101at 90° C, where the scheelite product is obtained with a minimum purity of 67 %.
To determine the pilot plant capacity, the generation rate of spent EN solution in the FES S. A. S. nickel plant, 750 L/week, was considered as the starting point. As it has been previously pointed out, the tungsten concentration in the spent solutions is variable and depends on operational conditions, among other factors. Therefore, an average tungsten concentration of 7813 mg/L was specified based on the AA analysis performed for different batches of solution waste. Additionally, a 100 % conversion of the tungstate into scheelite was considered as an overdesign element. With this information in hand, the average production of the pilot plant is projected to be 9178.2 g of scheelite per week. Also, to facilitate the operation and avoid the manipulation of excessive amounts of acid, two batches per day are proposed involving the treatment of 53.6 L of spent EN solution each 12 hours.
The reactor sizing stage considered the total volume of operation, the geometry and the volume change at the end of the chemical reaction. Since the process requires the use of highly corrosive substances, polyvinylidene fluoride (PVDF) was selected as the construction material due to its exceptional chemical resistance. However, only PVDF sheets are distributed by providers, implying that a rectangular geometry reactor must be constructed (Fig. 4). Regarding the volume change, experiments performed at the laboratory demonstrated that after the reaction is completed, no significant modification of the volume was observed. For practical purposes, this volume was assumed to remain constant. After the above-mentioned considerations and a 50 % overdesign element by stirring effects, the proposed total volume for the reactor R-101 is 171 L.
To ensure the formation of a more crystalline material in the reactor R-101 [16], the acid must be added slowly into the spent EN solution, according to the reaction shown in eq. (3).
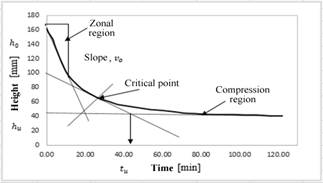
The mass balance for this reactor contemplates the mass of the spent EN solution (0.011 % sodium tungstate) (m
1
̇), the mass flow of nitric acid (m
2
) and the accumulation of mass between the final product and the unreacted materials
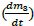 as shown in eq. (4). The minor mass loss ascribed to the release of acid gases was not considered.
as shown in eq. (4). The minor mass loss ascribed to the release of acid gases was not considered.
Based in the general energy balance (eq. (5)), the process within R-101 involved the internal energy of the spent solution (u
1
), the input enthalpy of the sodium tungstate (he
2
), heat of reaction (Q
rxn1) (between the tungstate and the acid), the heat of mixture between the acid and the solution a (Qmix1), nd the energy of the system due to accumulation
 as shown in eq. (6). The schematic global balance diagram for the reactor is depicted in Fig. 4.
as shown in eq. (6). The schematic global balance diagram for the reactor is depicted in Fig. 4.
It should be disclosed that the enthalpy and internal energy values of the spent solution were approximated to those reported for water, considering the overall composition (>70 % water). From eq. (6), the heat of mixture is calculated to be -19372 kJ leading to a final temperature of 56.4° C if the addition of acid occurs in a single step. To avoid this, and to maintain the reaction temperature below 30° C, heat extraction by direct aeration with cooled air at 5° C and a tube air diffuser is proposed. Moreover, the addition of nitric acid is planned to occur during a 3 h time lapse. The required mass of air (963.8 kg/3h) was calculated from eq. (7), where Q is the heat needed to be extracted from the mixture (19372 kJ), m is the mass of air (kg), C p the heat capacity of air 1.005 kJ/kg° C [16], and ∆T is the temperature difference of air inlet and outlet. This last one was established in 20° C.
The tungsten oxide settling velocity was identified as the main contribution that influences the decanter V-101 design. After the determination of this parameter in the laboratory, the type of sedimentation exhibited characteristics analogous to suspensions with a high concentration of solids with a well-defined limiting layer between the sedimented mass and the supernatant liquid [18,19].
From Kynch method [20], the decanter total area was calculated according to eq. (8), where A t is the total area of the decanter, 1.5 is an overdesign factor, ɸ is the hypothetic inlet flow rate (0.171 m³/h), t u is the experimental retention time 176.32 min, and h o is the initial vessel height 0.678 m. From these parameters, At for V-102 corresponds to 1.109 m².
On the other hand, the sludge area of the decanter depends on the operating time-lapse and the amount of sludge produced. To determine this area (clarification area Ac), eq. (9) was employed, where 2 is an overdesign factor, ɸ is the hypothetic inlet flow rate (0.171 m³/h), and vo is the slope of the zonal region (0.0059 m/min) observed in Fig. 5. The clarification area was calculated to be 0.96 m² (an additional heuristic overdesign factor of 30 % was also considered). It should be disclosed that the clarification area was proposed to have a rectangular pyramidal geometry (Fig. 6) based on the availability of the PVDF material and, as a strategy to improve the settling velocity.
Source: authors
Figure 5 Design parameters for V-101 obtained from the settling velocity curve of tungsten oxides
For reactor R-102, the total volume was established in 206 L, taking into consideration the average volume ratio of Ca(OH)2 0.1 M required and the tungsten oxide sludge separated from the decanter V-101. The mass balance in reactor R-102 considered the mass of the tungstic acid produced in the first reactor (m
4
), the amount of Ca(OH)2 necessary for the neutralization (m5), and the accumulation of mass between the final product and the unreacted materials
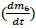 as shown in eq. (10).
as shown in eq. (10).
The heat balance in reactor R-102 involved the inlet enthalpy of Ca(OH)2 0.1 M (he5), the internal energy of the tungsten oxides (u4), the heat of mixture that included an estimated composition of water-HNO3 (Qmix2), the heat of reaction (Qrxn2), and the energy accumulation attributed to the addition of calcium hydroxide
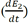 as shown in eq. (11) and Fig. 7.
as shown in eq. (11) and Fig. 7.
The heat of mixture in reactor R-102 was calculated according to eq. (11) to be -5160 kJ. However, this amount of energy can increase the temperature up to 30.7° C only. Thus, a cooling system was not contemplated for this stage.
The decanter V-102 design parameters were established using the same methodology and geometry of decanter V-101. Based on the settling curve for scheelite (Fig. 8), the total area and the clarification area were determined to be 1.14 m² and 0.047 m2, respectively.
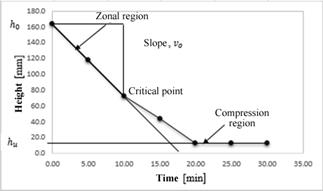
Source: authors
Figure 8 Design parameters for V-102 obtained from the settling speed curve of scheelite
After scheelite separation in the V-102 unit, an additional washing step is considered to remove soluble impurities that may have been adsorbed on the product surface. This treatment is proposed to involve 40 L of distilled water to wash the 36 L of scheelite precipitate employing an anchor-type stirrer. Once the washing operation has ended, the scheelite is again separated in V-102.
For the last step, the excess of water absorbed by the final product is removed in an electrical drying oven, which is planned to contain five racks, holding two 0.5m x 0.1m x 0.8m refractory glass trays. The total charge of the oven was dimensioned from the total mass of product obtained in the batches (simultaneous 72 kg of product and 36 kg as overdesign element), and considering that each batch requires three days at 90° C to achieve complete moisture removal, as indicated by the laboratory experiments.
Finally, after consideration of the above discussed elements of design for the scheelite production pilot plant, a 3D prototype of the plant to be installed in the FES S. A. S nickel plant is depicted in Fig. 9.
5. Conclusion
An innovative process for recycling of tungsten as scheelite from spent electroless nickel solutions was described. The implemented process proved to be efficient for the generation of scheelite product with a minimum purity of 67 %; this, when a non-analytical grade nitric acid was employed.
Moreover, the key plant design considerations were successfully established from laboratory scale experiments and basic operational parameters. The installation of this tungsten recycling plant is projected as a contribution to the national chemical industry and as an environmental strategy to reincorporate raw materials in the EN solution formulation.