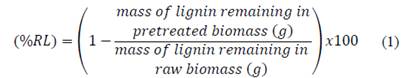1. Introduction
To contribute to mitigating global warming and environmental pollution, renewable energy production (REP) is promoted worldwide [1-6]. Agricultural residues such as sugarcane bagasse, African oil palm fiber, coffee pulp waste, and kikuyo grass can be converted into fermentable sugars, having the potential to produce biofuels, high-value bioproducts, and second-generation bioethanol [7-14]. These lignocellulosic residues are produced in large amounts and are generally discarded in tropical countries like Colombia. However, some efforts are currently being directed towards their utilization, requiring further research to support and confirm their commercial potential [8]. Cellulose, hemicellulose, and lignin are the main compounds of these vegetable materials. Sugars contained in cellulose and hemicellulose fractions, for instance, can be fermented to produce bioethanol [15].
For the processing of these wastes, pretreatments are usually required [9,10,12], these consist of a disruption of the lignocellulosic matrix, reduce the hemicellulose and lignin content and modify the crystalline structure of cellulose. Higher susceptibility to enzymatic attack can be achieved [9]. This process becomes an important factor to produce a suitable source of fermentable substrate [12]. Within bioethanol production, pretreatment represents the second most expensive step. Thus, finding an appropriate technology is essential [10]. Physical, chemical, physicochemical, and biological pretreatments are currently applied, but its choice must consider certain criteria: preservation of hemicellulose and cellulose fraction, cellulose decrystallization, low production of toxic compounds potentially inhibitory for fermentative microorganisms, increase in accessible surface area, solubilization of hemicellulose and/or lignin, increasing of fermentable sugar yields after hydrolysis, cheaper and environmentally friendly reagents, and catalyst recovery and/or solvent recycling [10,16]. Unfortunately, none of the pretreatments meets all these criteria at the same time [1].
Alkaline pretreatment is considered an effective chemical pretreatment for the delignification of agricultural wastes [10,2,17]. Among the desirable features, it can operate under mild temperature and pressure conditions with minimal sugar degradation and without the formation of inhibitory compounds, solubilizing mainly lignin to enhance the cellulose content and improving carbohydrate digestibility [1,7,9,15,18]. Non-polluting and non-corrosive reagents such as ammonia, sodium hydroxide, sodium carbonate, and calcium hydroxide are used. Although the effect with calcium hydroxide is not as strong as that with the other reagents, its low cost, safe handling, and simple recovery make it an attractive option [17,18]. Previous studies on alkaline pretreatment have shown its effectiveness in a variety of modes and conditions with treatment times on the order of hours and days rather than minutes or seconds. Most of the acetyl groups and about 10-52% of the lignin have been removed, enhancing enzymatic saccharification on sugarcane bagasse, switchgrass, corn stover, poplar wood, wheat straw [9,17,19-25]. Although this pretreatment has been broadly studied on different types of lignocellulosic biomass, there is not a comparative study regarding the use of sugarcane bagasse, African oil palm fiber, coffee pulp waste, and kikuyo grass to provide fermentable and scalable syrups.
The substrate structure and composition of lignocellulosic biomass as well as pretreatment conditions are important factors that determine the effectiveness of pretreatment [9]. This study aimed to assess the effect of alkaline pretreatment on the structure, composition, and subsequent hydrolysis of selected agricultural wastes to produce fermentable sugars that may be substantially important in the production of cellulosic ethanol during the bioconversion process.
2. Materials and methods
2.1 Materials
Sugarcane (Saccharum Officinarum L.) bagasse, African oil palm fiber (Elaeis Guineensis), coffee (Coffea Arabica L.) pulp waste, and kikuyo grass (Panisetum Clandestinum) were collected, oven-dried at 45°C for 72 hours, cooled at room temperature (25°C) for 24 hours, milled, and screened out with 16-50 mesh sifter. These samples were then stored in zipper plastic bags and placed in a refrigerator at 4°C until further testing [26]. The lignocellulosic materials were provided by farms in the vicinity of the Universidad Nacional de Colombia, Medellin. For experimentation, the following reagents were used: Calcium hydroxide reagent grade (Fisher Scientific, United States), sulfuric acid analytical grade (Merck Millipore, Germany), commercial cellulase/hemicellulase Multifect CX GC (Genencor, United States), and Multifect CXB (Enmex, Mexico) from Trichoderma reesei. Tween 80 (Fisher Scientific, United States), acetic acid, and sodium acetate reagent grade (Merck Millipore, Germany).
2.2 Pretreatment
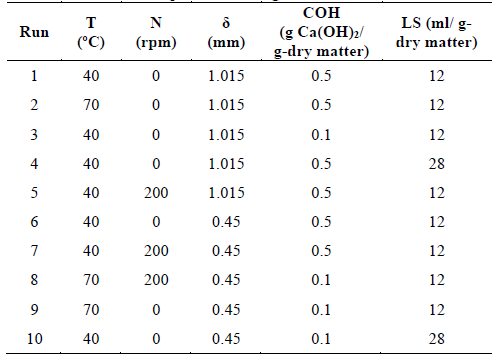
Twenty samples (dry matter) of each biomass were placed in autoclaved 225 ml Erlenmeyer flasks, corresponding to duplicate treatments. Distilled water was used to achieve the liquid to solid ratio of the experimental design shown in Table 1. Two temperature levels (40 and 70°C), two alkali concentrations (0.1 and 0.5 g Ca(OH)2/g-dry matter), two liquid to solid ratio levels (12 and 28 ml/g-dry matter), two stirring rate levels (0 and 200 rpm), and two average particle size levels (0.45 and 1.015 mm) were considered. The removal of lignin (%RL) from the treated biomass was defined as the response variable. The pretreatments were conducted for 24 hours. The evaluated variables and levels were selected according to the literature [19,20,24-29]. After the pretreatment time elapsed, the Erlenmeyer flasks were immersed in cold water to cool them. The pretreated biomass was filtered out and washed with distilled water until the pH was between 7 and 8. Then, they were dried at 45°C to constant weight for 72 hours. Subsequently, these samples were cooled at room temperature and stored at 4°C in zipper plastic bags [26,30,31]. The resulting pretreated material was mainly characterized by determining the moisture and lignin content.
2.3 Hydrolysis
After selecting the optimal conditions for pretreatment, calcium hydroxide-pretreated biomass was transferred to autoclaved 250 ml Erlenmeyer flasks. Pretreated sugarcane bagasse, African oil palm fiber, and coffee pulp waste were hydrolyzed with dilute sulfuric acid while pretreated kikuyo grass was enzymatically hydrolyzed. Control samples of untreated biomass were also included. Experimental conditions were selected according to literature and preliminary evaluations. For hydrolysis of sugarcane bagasse, the acid concentration (4.2% v/v), liquid to solid ratio (7.7 ml-liquor/g-dry matter), temperature (94°C), and reaction time (24 hours) were considered [27-29]. For the hydrolysis of African oil palm fiber, the acid concentration (3% v/v), liquid to solid ratio (8 ml/g), reaction time (3 and 24 hours), and temperature (94°C) were assessed [30-32]. Similarly, for the acid hydrolysis of coffee pulp waste, the acid concentration (2% w/v), liquid to solid ratio (10:1 ml-dilute acid/g-dry waste), stirring rate (335 rpm), reaction time (4 and 6 hours), and boiling temperature with reflux were used [9,33]. On the other hand, the enzymatic hydrolysis of kikuyo grass was carried out using the enzymatic complex Multifect CX GC/Multifect CXB with concentrations of 1.036% and 0.736% w/w, respectively. The operational conditions included liquid to solid ratio (16.7 ml-buffer/g-dry matter), temperature (50°C), sodium acetate/acetic acid buffer solution (pH 5.08), stirring rate (180 rpm), tween 80 (5 g/l), and reaction time (72 hours) [8,14,34]. After the reaction time elapsed, the Erlenmeyer flasks were immersed in cold water, cooled down to ambient temperature. The hydrolyzed materials were then centrifuged at 2500g for 10 min. The supernatant was removed and neutralized with sodium hydroxide (4M). Aliquots were retrieved and diluted (10% v/v) for the determination of reducing sugars (RSs).
2.4 Analytical methods
An initial characterization of the lignocellulosic materials was performed in the Laboratory of Chemical and Bromatology Analysis at the Universidad Nacional de Colombia, Medellin as shown in Table 2. Analysis for lignin, cellulose, hemicellulose, starch, sulfur, potassium, phosphorus, calcium, sodium, protein, moisture and ash content were carried out using standard protocols [40,41]. Reducing sugars (RSs) were measured using the dinitrosalicylic acid (DNS) assay. A glucose standard solution was used for calibration [42]. The absorbance readings of the samples were obtained with a spectrophotometer at 540 nm [13,14]. Additionally, the lignin content of the untreated and treated biomass was determined using the acetyl bromide spectrophotometric method as a quick and easy approach to determine its total content in agricultural samples [43,44]. The sample preparation for analysis and extraction procedures were performed according to the Laboratory Analytical Procedure, NREL [45]. All determinations were conducted by duplicate [7]. Reported data in tables and figures correspond to average values with a relative standard deviation of less than 5%. The removal of lignin (%RL) from the pretreated biomass was defined through Eq. (1) [14,46].
The morphology and physical structure of the lignocellulosic materials, before and after pretreatment, were analyzed using Scanning Electron Microscope (SEM, Hitachi S-4000, UK) with an accelerating voltage of 10kV. The changes in the external structures caused by the pretreatment were identified. Samples were dried and mounted on double-sided carbon tape on brass sample stubs, and sputter-coated with 30 Å of Au/Pd film. All images were generated at 250x and 1000x magnification.
2.5 Statistical analysis
Graphical analysis and rudimentary statistical assessments, such as variance analysis (ANOVA) of a single factor, were used to identify trends and levels of significance or impact on the lignin content of the studied lignocellulosic materials. The alkali concentration, liquid to solid ratio, particle size, temperature, stirring rate, and biomass type were investigated for the alkaline pretreatment. The statistical software packages STATGRAPHICS Centurion VII® (version 17.2.00, 64-bit) and MINITAB® (version 19.1, 64-bit) were implemented.
3. Results and discussion
3.1 Characterization of lignocellulosic materials
The composition of the lignocellulosic materials used in this study is shown in Table 2. The main fractions of sugarcane bagasse were in the range of reported content for lignin (10-28%), cellulose (20-46%), and hemicellulose (20-33%) of the same material [5,27,29,42,43]. For African oil palm fiber, similar contents of lignin and cellulose were found to those values reported of palm oil solid wastes. Lignin (10-28.8%), hemicellulose (17-22.8%), and a higher percentage of cellulose (36.7-54.4%) than other sources of biomass such as sugarcane bagasse, rice straw, and corn cobs have been registered [10,30,31,44-46]. Regarding the composition of coffee pulp waste, the main fractions of lignin and cellulose were in the range of lignin (11.7-16.85%) and cellulose (8.6-53%) contents respectively, but the hemicellulose content (0.1%) was lower than the reported values (11-36.7%) [2,9,33,47]. Finally, the kikuyo grass had a higher content of lignin, cellulose, and hemicellulose than the values found for the same biomass. The lignin (5.6-5.9%), cellulose (26.9%), and hemicellulose (26.2%) contents were reported [8,13]. Variations in the composition can be related to different growing conditions, species, times of harvesting, processing of samples, among others [14]. From this initial characterization, the content of cellulose and hemicellulose highlights the potential of the selected agricultural residues to produce fermentable sugars, bioethanol, and other high-value bioproducts through their fractionation or hydrolysis. For instance, theoretical ethanol yields can be broadly estimated to show their big potential [57].
Table 2 Composition of lignocellulosic materials.
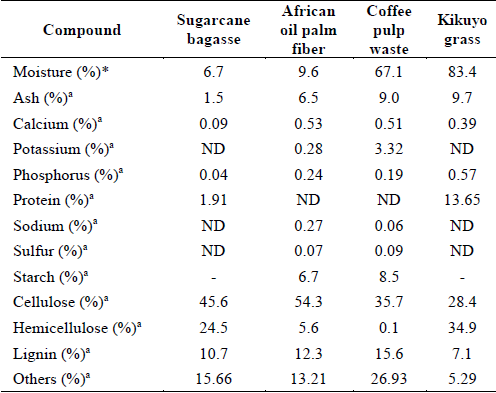
ND: not determined, *raw basis, a dry basis.
Source: The authors.
The values of 504.56, 455.57, 431.20, 257.73 l/ton-dry matter were computed for the sugarcane bagasse, kikuyo grass, African oil palm fiber, and coffee pulp waste, respectively. The implementation of alkaline pretreatment is therefore suggested to reduce the lignin content and enhance the subsequent hydrolysis and products yields, among other benefits.
3.2 Alkaline pretreatment
Table 3 shows the percentage of lignin loss (%RL) after the pretreatment process. The optimal pretreatment parameters and overall trends can be identified for each lignocellulosic material.
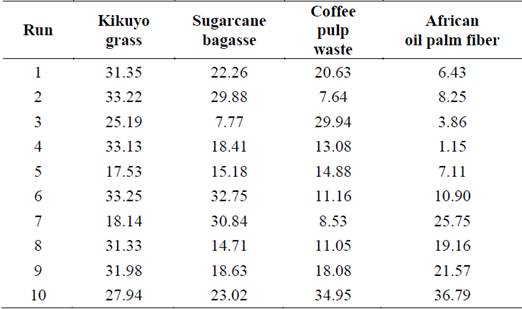
From the results of Table 3, the treatment process that provided the highest removal of lignin was 10 (36.79%) for African oil palm fiber when the lowest level of factors: temperature (40 ºC), stirring rate (0 rpm), average particle size (0.45 mm), and alkali concentration (0.1 g Ca(OH)2/g-dry matter) were assessed at the high liquid to solid ratio (28 ml/g-dry matter). A similar result (34.95%) was obtained when coffee pulp waste was treated under the same operational conditions. On the other hand, the highest removal of lignin from kikuyo grass (33.25%) and sugarcane bagasse (32.75%) was achieved using the lowest level of temperature, stirring rate, average particle size, and liquid to solid ratio (12 ml/g-dry matter) while the alkali concentration had the highest value (0.5gCa(OH)2/g-dry matter), corresponding to the treatment process 6.
As observed, the lowest lignin loss (1.15%) was obtained with African oil palm fiber under the treatment 4 while the other lignocellulosic materials showed higher removal of lignin than 13.08% for the same experimental condition. Overall, there was a percentage of removal of lignin for each treatment and biomass, suggesting a certain dependency on the tested material. [14] reported the minimal (20.74%) and maximum (76.73%) percentages for the removal of lignin from coffee pulp, respectively. The minimal value was obtained using an autoclave as the reactor at 121ºC with Ca(OH)2 (4% w/v) for 25 min. To achieve the maximum removal of lignin, NaOH (6.37 %w/v), Ca(OH)2 (6.37 %w/v), and 30 min of reaction were used with an autoclave. A liquid to solid ratio (5 ml/g-raw matter) was considered. [53] assessed the alkaline delignification of oil palm empty fruit bunch fiber and compared the effect of including an autoclave during the treatment. Using 2 mm fiber length, NaOH (0.5%v/v), liquid to solid ratio (10 ml/g-raw matter) at 30ºC for 4 hours without autoclaving, no removal of lignin neither significant changes of cellulose nor hemicellulose composition were observed. When the autoclave was included (121ºC, 15 psi for 5 min), the cellulose increased its content, and the removal of lignin was 17%. Subsequently, [50] also treated oil palm empty fruit bunch fiber and assessed different concentrations of NaOH (0.5 and 2% v/v). The complementary experimental conditions were as those of their previous work. A similar effect was described when the autoclave is used during the treatment. The removal of lignin reached 63% and hemicellulose was also removed using NaOH (2% v/v). It was inferred that autoclaving can also alter the physical structure of lignin since its oxidation and hydrolysis may be easily carried out. As lignin dissolves in alkali media, expanding its surface, higher accessibility for oxidation can be present. Hemicellulose is also soluble and is expected to be less stable than cellulose due to its branched structure.
[20] used alkaline pretreatment with sugarcane bagasse. The amount of 14% lignin became solubilized. The hydroxide loading (0.1 g Ca(OH)2/g-dry matter), short reaction times (1-3 hours) with high temperatures (85-135ºC), and long reaction times (e.g. 24 hours) with low temperatures (50-65ºC), liquid to solid ratio (10 ml/g-dry matter), average particle size (0.4 mm) were recommended to enhance the digestibility. [10] also evaluated the effect of alkaline pretreatment on sugarcane bagasse and removed 30% of lignin using 0.1 g Ca(OH)2/g-dry matter for 1 hour at 120ºC. Low solubilization of hemicellulose (5%) accompanied by a cellulose accumulation (11%) was obtained. [19] assessed the alkaline pretreatment to improve the digestibility of switchgrass. Different pretreatment conditions were explored to determine the effects of process variables (i.e. temperature, calcium hydroxide loading, liquid to solid ratio, and biomass particle size). Fairly large quantities of lignin and a low fraction of other compounds (e.g. xylan-hemicellulose, crude protein) were dissolved. The amount of lignin removed was 29.4% at 120ºC, 2 hours, 0.1 g Ca(OH)2/g-dry matter, liquid to solid ratio of 9 ml/g-dry matter, and particle size less than 0.4 mm. The removal of lignin significantly contributed to increasing biomass digestibility. [54] treated coastal Bermuda grass with NaOH (1%, equivalent to 0.1 g/g-dry matter at the solid loading of 10%) and Ca(OH)2 (0.1 g/g-dry matter) at 121ºC, 30 min and particle size 2 mm. About 75% and less than 20% of lignin removal were found, respectively.
The obtained results for the removal of lignin from African oil palm fiber, coffee pulp waste, kikuyo grass, and sugarcane bagasse are comparable to those values reported by other studies. Note that mild conditions were assessed herein. It is corroborated that alkaline pretreatment had an important effect on delignification. A small dissolution of hemicelluloses with an insignificant reduction in cellulose content is desired [10,18,25]. In fact, no degradation of the cellulosic fraction results in a higher concentration of fermentable sugars after hydrolysis, which is a relevant aspect for the economic viability of the conversion process [10].
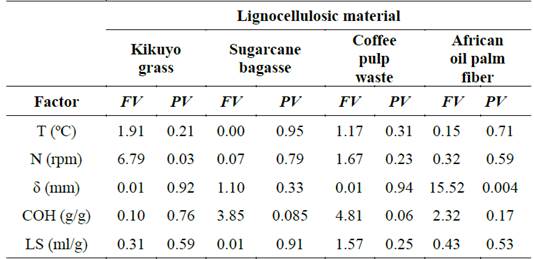
The variance analysis (ANOVA) of a single factor for the %RL is shown in Table 4. This analysis allowed us to identify factors of significance or impact on the alkaline delignification of the lignocellulosic materials.
As shown, the particle size (δ) of the African oil palm fiber and the stirring rate (N) used during the treatment of kikuyo grass were statistically significant at the level of 5% significance (P < 0.05) with F values of 15.52 and 6.79 (corresponding to Fisher’s F test), respectively. The other factors such as temperature (T), alkali concentration (COH), and liquid to solid ratio (LS) were not statistically significant and may be assumed of minor relevance for the treatment of each biomass. Nonetheless, low water loading may result in a very thick paste that would cause handling and mass transfer problems. Pretreatment time and temperature have shown a certain dependency. Short reaction times (e.g. 1 hour) generally require high temperatures (e.g. 125ºC) for effective pretreatment. A critical hydroxide loading (0.1 g Ca(OH)2/g-dry matter) has been suggested [19,20]. Biomass digestibility can be improved by removing acetate groups from hemicellulose when enough calcium hydroxide is added, but further addition might be considered ineffective [19,20].
On the other hand, when the biomass type was included as a factor in the statistical analysis, the P-value (0.006) and F value (4.87) were computed, meaning that there was a statistically significant difference between the mean of removal of lignin from one level of biomass to another. On average, kikuyo grass had the highest %RL of 28.31% followed by sugarcane bagasse (21.35%), and coffee pulp waste (16.99%) while African oil palm fiber had the lowest value (14.10%). Each biomass material exhibited a unique behavior under the evaluated operational conditions.
The main effects of factors on the removal of lignin with lignocellulosic materials are depicted in Fig 1. Fig 1a shows the positive effect of temperature and liquid to solid ratio on the delignification of kikuyo grass when using the highest level of these factors. Similarly, this positive effect can be observed at the lowest level of the stirring rate, particle size, and alkali concentration.
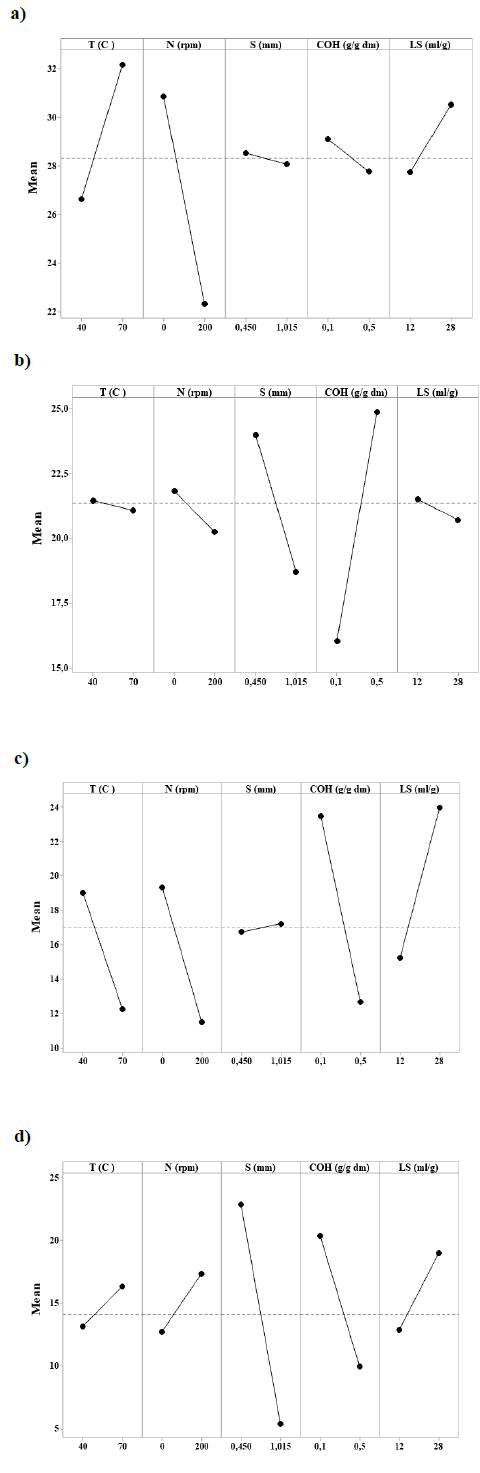
Source: The authors.
Figure 1 Main effects of factors on the removal of lignin with lignocellulosic materials. a) Kikuyo grass, b) Sugarcane bagasse, c) Coffee pulp waste, and d) Fiber of African oil palm. Where “dm” refers to dry matter, and “S” corresponds to the particle size (δ) used in Table 1.
However, the most significant factor was attributed to being the stirring rate. Fig 1b represents the effects of factors on the delignification of sugarcane bagasse. A positive effect is depicted at the highest level of alkali concentration. High removal of lignin is observed at the lowest level for temperature, stirring rate, particle size, and liquid to solid ratio. Fig 1c shows a positive effect of the liquid to solid ratio and particle size on the delignification of coffee pulp waste when these factors were evaluated at their highest level while a negative effect is observed for the other factors. Finally, the negative effect of the particle size and alkali concentration on the delignification of the African oil palm fiber when they were evaluated at their highest level is shown in Fig 1d. The particle size was identified as the most significant factor for the alkaline treatment of this biomass. A positive effect was observed when the other factors were assessed at their highest level. As shown in these plots, each factor had different effects on the alkaline pretreatment of the tested materials. They did not follow an overall trend.
3.3 Structural analysis in the pretreated lignocellulosic materials
The structure and composition of lignocellulosic biomass, as well as pretreatment conditions, are important factors that determine the effectiveness of pretreatment [10]. When optimal conditions are used, the improvement of digestibility as the action of the calcium hydroxide is relevant [19]. Calcium ions extensively cross-linked lignin molecules, disrupting chemical bonds stiffening lignocellulose by removing lignin and acetyl groups from hemicelluloses. This can lead to increase the porosity and effectively improve the digestibility of pretreated material [10]. Modifications on the surface of assessed lignocellulosic materials were analyzed by Scanning Electron Microscopy (Fig 2).
Kikuyo grass (Fig 2a-2) and sugarcane bagasse (Fig 2b-2) were pretreated using the lowest level of temperature (40°C), average particle size (0.45 mm), liquid to solid ratio (12 ml/g-dry matter) without agitation, and the highest hydroxide concentration (0.5 g Ca(OH)2/g-dry matter). Coffee pulp waste (Fig 2c-2) and African oil palm fiber (Fig 2d-2) were pretreated at the lowest level of temperature, stirring rate, average particle size, hydroxide concentration (0.1 g Ca(OH)2/g-dry matter), and the highest liquid to solid ratio (28 ml/g-dry matter). These pretreatments were conducted for 24 hours.
From Fig 2, it is observed that all materials maintained their tissue integrity to some extent. Nevertheless, signs of fragmentation appear on the surface of pretreated samples, and the possible formation of calcium carbonate deposits that may minimize the porosity of pretreated coffee pulp waste was evident (Fig 2c-2). Lignin and carbohydrate degradation products from the pretreatment tend to degrade all the way to carbon dioxide forming carbonates in the alkaline medium [55]. A cellular matrix with ordered and compact structure is observed for untreated materials, while pretreated samples (i.e. kikuyo grass, sugarcane bagasse) showed damage in their structure which included rupture of cell walls, exposing inner parts, as well as increasing the surface area and making them more accessible to subsequent hydrolysis. These observations agree with reported results from other studies [1,7,10]. As seen, pretreated African oil palm fiber (Fig 2d-2) exhibited a rough surface due to the orientation changing of highly packed crystalline cellulose order to form an amorphous region, which allowed the OH group to break down to react with water molecules and then leave the fiber structure. Besides reducing the hydrophilicity of fiber, removal of lignin and other possible compounds (e.g. wax, hemicellulose, and oil that has coating the fiber surface) could have taken place [56].
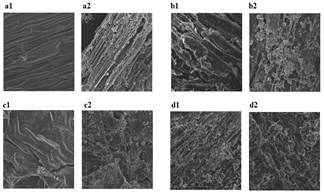
Source: The authors.
Figure 2 Scanning electron microscopy of lignocellulosic materials (a. Kikuyo grass, b. Sugarcane bagasse, c. Coffee pulp waste, and d. Fiber of African oil palm) without pretreatment (1) and pretreated with calcium hydroxide (2).
Two levels for destructuring the cell walls of pretreated biomass have mainly been attributed to lignin removal. The first level corresponds to the loss of cohesion between neighboring cell walls and the second level refers to degradation inside the cell wall caused by peeling and formation of holes [10,57]. The disruption of fibers that occurred to the assessed materials (e.g. sugarcane bagasse) after alkaline pretreatment was likely due to the removal of lignin which would enhance the hydrolysis of cellulose into reducing sugars (RSs) as shown in the following section. In fact, changes in the structure and morphology subjected to the pretreatment were visible, confirming that part of the lignin fraction was removed since it is responsible for the highly compact structure of plant cell walls [46].
3.4 Hydrolysis of lignocellulosic material
To assess the pretreatment efficiency and verify whether the cellulose was available in the pretreated material, each biomass was hydrolyzed. Pretreated sugarcane bagasse, African oil palm fiber, and coffee pulp waste were hydrolyzed with dilute sulfuric acid while pretreated kikuyo grass was enzymatically hydrolyzed. Table 5 presents the concentration (g/l) of reducing sugars (RSs) obtained during and/or after hydrolysis. Control samples of each untreated biomass were also included.
Table 5 Results for hydrolysis. Reducing sugars (g/l).
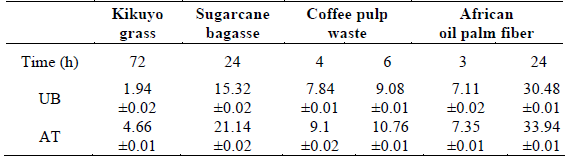
UB: untreated biomass. AT: alkaline-treated biomass.
Source: The authors.
There is a relationship between the cellulose content and the amount of reducing sugars (RSs) produced once the alkaline pretreatment is applied. Table 5 shows an increase in the content of reducing sugars for each of the pretreated biomasses. For instance, there is an increase from 30.48 ±0.01 to 33.94 ±0.01g/l for African oil palm fiber, and there is an increase from 15.32 ±0.02 to 21.14 ±0.02 g/l for sugarcane bagasse. This is associated with the cellulose content of 54.3% and 45.6% for African oil palm fiber and sugarcane bagasse, respectively. The above confirms that the characterization of these materials is an important starting point for their valorization. Although this does not completely confirm the effectiveness of alkaline pretreatment as the analysis of the percentage of delignification does, it allows proposing a framework and processing route for the generation of products of interest.
As observed, the amount of sugar produced after hydrolysis from pretreated kikuyo grass, sugarcane bagasse, and African oil palm fiber was higher than the control samples, suggesting the relevance of their pretreatment to remove or decrease the lignin and likely hemicellulose content that form a physical barrier involving cellulose in the lignocellulosic material. The alkaline pretreatment was mainly efficient for the removal of lignin, and it was not necessary to remove this compound up to 60-80% to enhance sugar production. A similar outcome has already been observed by other authors [14,54].
Although coffee pulp waste had a significant %RL of 34.95%, there was not a big difference between the sugar production from the pretreated material and control sample after 6 hours of reaction. A negative effect of calcium hydroxide in RSs concentration and pretreatment efficiency has been reported after enzymatic hydrolysis of this biomass [14]. As shown in Fig 2c-2, it may be hypothesized that the formation of calcium carbonate deposits may have protected cellulose in the places where there had already been degradation, thus forming a protective layer that prevented further hydrolysis of carbohydrates [50]. Alternatively, the presence of xenobiotics such as caffeine, tannins, free polyphenols, and other pollutant agents, characteristic of this biomass, may have had a detrimental effect [7]. [38] registered the glucose concentration of 5.66 g/l with untreated coffee pulp using dilute sulfuric acid (2 %w/v), 4 hours, boiling temperature with reflux, a liquid to solid ratio of 10:1, and particle size less than 1 mm.
Regarding sugarcane bagasse, [34] obtained a solution with 21.6 g xylose/l and 3 g glucose/l using sulfuric acid (2 g H2SO4/100 g liquor), a liquid to solid ratio of 10:1 at 122ºC for 24 min and untreated material of 0.5 mm particle size. [33] achieved the RSs concentration of 16.76 g/l with non-treated bagasse pith at room temperature, sulfuric acid (6 % v/v), a liquid to solid ratio of 31:1, and 4 hours of reaction. [58] produced a concentration of sugars (i.e. glucose, xylose) between 35.2 and 39.4 g/l after 8 hours of enzymatic hydrolysis using engineered biomass (lignin-reduced sugarcane) while 26.6-29.2 g/l were obtained using wild type biomass.
[35] found concentrations of xylose (29.4 g/l) and glucose (2.34 g/l) from batch hydrolysis of untreated oil palm empty fruit bunch fiber, which was performed at 120ºC using sulfuric acid (6 g H2SO4/100 g liquor), a liquid to solid ratio of 8:1, particle size less than 1 mm, and reaction time (15 min). Similarly, the concentrations of xylose (4.48 g/l) and glucose (7.61 g/l) were obtained at 130ºC, 6 g H2SO4/100 g liquor, a liquid to solid ratio of 8:1, and 90 min in their subsequent work using the same lignocellulosic material [36]. [49] obtained the reducing sugar concentration of 17.8 g/l after hydrolysis of pretreated fibers (NaOH 0.5M, 121ºC, and 12 min) using particle size less than 1 mm, 5% solids, 300 rpm, 10% HCL, at 45ºC and 1 hour.
Considering the hydrolysis of kikuyo grass, [13] reported the greatest RSs concentration of 2.53 g/l after an aqueous pretreatment by immersion in distilled water (liquid to solid ratio of 1:5 for 1 hour at ambient temperature). A sulfuric acid solution (4 %v/v), the liquid to solid ratio of 30:1, boiling temperature with reflux, and 6 hours were used for acid hydrolysis. Through enzymatic hydrolysis, reducing sugars production was not significant (0.053 g/l) after 6 hours of reaction with the water-cellulose mixture (90 ml - 0.03 ml Celluclast 1.5 LFG) at ambient temperature.
Even though high sugar concentrations are desired, an exerted osmotic pressure on yeasts may lead to plasmolysis and lower ethanol production during fermentation [39]. An increase in the total sugars from 40 to 80 g/l has shown a negative effect on ethanol yields by using the co-culture of S. stipitis and S. cerevisiae [59]. The concentrations of fermentable sugars, obtained herein from the pretreated lignocellulosic materials, were placed between the amounts reported by other investigators and can be considered suitable to produce cellulosic ethanol. Estimated ethanol yields for the pretreated sugarcane bagasse (105.22 l/ton-dry matter), pretreated kikuyo grass (50.30 l/ton-dry matter), pretreated African oil palm fiber (175.51 l/ton-dry matter), and pretreated coffee pulp waste (69.55 l/ton-dry matter) highlight their big potential [57]. Additional experimentation can provide major insights by comparing these theoretical yields. Future work will be performed, verifying the presence or absence of inhibitors with the obtained fermentable sugar syrups, enabling its production at a large scale.
4. Conclusions
This study suggests that the assessed lignocellulosic materials (i.e. kikuyo grass, sugarcane bagasse, African oil palm fiber, and coffee pulp waste) are proper sources for producing fermentable sugars, which may lead to the production of second-generation bioethanol through the identification and selection of correct conditions for pretreatment and hydrolysis. As well as factors, trends, and levels of significance or impact on the delignification of these materials were elucidated. The use of calcium hydroxide is considered as an efficient pretreatment method of kikuyo grass, sugarcane bagasse, and African oil palm fiber to enhance sugar production. This approach can be economical, safe, environmentally friendly, easy-to-use, and easy-to-recover for bioethanol production and other industrial applications [19].
The removal of lignin was strongly related to the biomass type. Each factor presented different effects on the alkaline pretreatment of the agricultural materials. The average particle size was a statistically significant factor for the pretreatment of African oil palm fiber while the stirring rate was an important factor during the pretreatment of kikuyo grass. The calcium hydroxide concentration, on the other hand, had a major effect on the delignification of sugarcane bagasse and coffee pulp waste.
After hydrolysis, the highest concentration of reducing sugars (RSs) was obtained with the pretreated biomass over a relatively short time interval. Concentrations of 21.14, 10.76, 33.94, and 4.66 g/l were measured for sugarcane bagasse, coffee pulp waste, African oil palm fiber, and kikuyo grass, respectively. This is associated with the cellulose content of these materials, 45.6, 35.7, 54.3, and 28.4%, respectively.
Morphological changes were observed using Scanning Electron Microscopy (SEM). The captured surface morphology revealed damage to the structure of pretreated biomass materials, which would be useful to increase the porosity making the carbohydrates more accessible by enzymes or chemicals.
The obtained results reinforce the feasibility of implementing the alkaline pretreatment to reduce the lignin content and enhance the subsequent hydrolysis of agricultural wastes. A suitable production of fermentable sugars was achieved with the procedure and protocols used here. Mild conditions were assessed which may be economically important in the production of cellulosic ethanol.