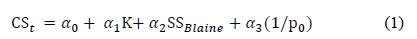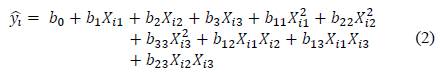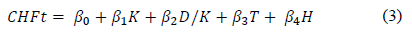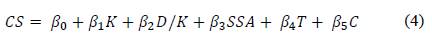1. Introduction
The building and infrastructure projects require management of large volumes of materials such as excavated soil and rocks, which are generated when digging in the ground [1], large volume of construction building materials, and finally generate a large amount of wastes from demolition processes. The excavated soil can be reused at the same construction sites and in the restoration of other; however, the majority of the excavated soil is dumped in landfills generating negative environmental impacts [2]. Similarly, the demolition waste can be used as supplementary cementing materials or aggregates in pastes, mortars and concrete [3-5].
In the case of the Bogotá city (Colombia), the soil is formed by lacustrine deposits and its mineralogical composition includes as much as 50 % quartz, 15 - 42 % feldspars and 15 - 30 % clay [6]. Some mining by-products made up of clays have been valued by being used as supplementary cementitious materials, contributing to their environmental management [7,8], since it is possible to remove structural water by thermal treatments obtaining an amorphous phase with pozzolanic activity as has traditionally been done with kaolinitic clays [9-11] and how is being done with multicomponent clays [12-14].
Based on the results obtained by other researchers with kaolinitic clays, multicomponent clays and with industrial waste made up of clays, some researchers have begun to evaluate the pozzolanic potential of excavated waste clays. Priyadharshini et al [15], Zhou et al [2] and Lorentz et al [16] characterized excavated clays and indicated that are composed mainly of clay minerals like kaolinite, illite and montmorillonite. Zhou et al [2] discovered that the calcined excavated waste clays at 800, 900, and 1000 °C show pozzolanic activity after 8 and 15 days of cured based on the Frattini test and strength activity index (SAI) results. It was concluded that pozzolanic activity of excavated waste clays depend on calcination temperature, kind and quantity of clay minerals and impurities. Du and Pang [17] calcined a marine clay collected from a construction site in Singapore, with a kaolinite content approximate of 20%. Mortars with marine clay calcined at 600 °C exhibited a high strength activity index of approximately 0.9, despite the low kaolinite content.
Some previous studies related the pozzolanic activity of different kaolin with the characteristics that influence their behaviour, using linear regression models. Garg and Wang [18] compared the performance of three different commercially available clays calcined and conclude that the clays with kaolinite were the most reactive. Rong et al [19] studied mortars with 10% calcined kaolinite clay and determined the highest compressive strength and flexion compared to the others, due to the pozzolanic reaction produced by the strengthening of the interface and the densification of the mortar microstructure. Güneyisi et al [20] assessed the statistical significance of kaolin from different quarries and calcination temperature through general linear model analysis of variance. The effective test parameters on pozzolanic activity index were determined; samples with kaolinite contents around 54% calcined at 750 °C, reached the maximum values (107%). The p-value of the parameters were less than 5% indicating that, the type of kaolin and the calcination temperature are both significant on the pozzolanic indices of mortars.
Mermerdas. et al [21] investigated the effectiveness of calcined kaolins from different deposits, replacement level and age on the compressive strength of concrete through general linear model analysis of variance. The highest relative strength values were achieved by sample with highest kaolinite content, at 15% replacement level. The effects of all parameters were determined be statistically significant since the p-values are less than 0.05.
Tironi et al [22] proposed different models to relate the compressive strength at different ages with the composition, degree of structural disorder and specific surface of five kaolinitic calcined clays. The best fit was a linear combination of the compressive strength at time t (CSt) with kaolinite content in clay (K), the specific surface area (SSBlaine) of calcined clay and the inverse of the index that provides the degree of order/disorder (1/P0) of the structure before calcined kaolinite as the equation:
Samet et al [23] studied the effect of calcining temperature (X1), the specific surface area of calcined clay (X2) with 72 % kaolinite content and its percentage in the blended cement (X3) on the compressive strength of mortars, through a second order polynomial function:
where Xij is the value of coded variable j at the ith experiment, b j and b ij are model coefficients, ŷ t is the calculated compressive strength at 7, 28 and 90 days.
Most studies have focused on kaolin, kaolinitic clays and commercial kaolin that have been thermally activated in order to generate pozzolanic activity. There is a consensus in the literature about the effects of factors such as kaolinite content, calcination temperature and curing age on the compressive strength. However, there is still a need for knowing and propose a model to quantify the effect of these characteristics among others on the compressive strength and even the fixation of calcium hydroxide of excavated waste calcined clays with presence of different polymorphs of kaolinite group: kaolinite and dickite.
In that regard, the aim of this work is to quantify the effect of excavated clays characteristics with kaolinite group minerals content on the compressive strength and even the fixation of calcium hydroxide, to determine how is influenced their performance as supplementary cementitious materials. In this study, five samples of excavated waste clays were thermally activated using two calcination temperatures in order to evaluate the effect of the kaolinite and dickite contents, the specific surface area, the calcination temperature, and the curing age on the pozzolanic activity in lime pastes and blended mortars.
2. Materials and methods
2.1 Materials
Excavated waste clays (A1-A5) were selected from different soft soil deposits of Sabana Formation (Quaternary in age) according to Bogota geological information FOPAE [24] and in accordance with the places where the biggest construction and demolition waste generators are.
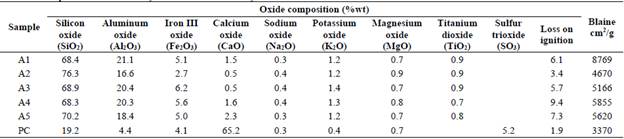
Portland cement without additions was used for making mortars and its chemical composition is related in Table 1.
2.2 Characterization of raw excavated clays
The particle size distribution of excavated clay was carried out in a Mastersizer 3000 and the Blaine method was used in order to calculate the specific surface area (SSA), according to ASTM C204 [25]. The chemical composition of the A1 - A5 clays was determined by X-ray fluorescence (XRF) such as is detailed in our previous paper [13], and the results are shown in Table 1.
The X-ray diffraction patterns were identified by a Panalytical X'pert PRO, with CuKa (1.54 Å) radiation in the range of 3° - 60° (2θ) at 45 kV and with powder diffraction search manual - COD2016 database. FWHM - 001 index was determined as the width at half height of the 001 peaks of kaolinite, range from >0.4 (disordered) to <0.3 (ordered) [26]. FTIR spectrums were obtained using a Nicolet Magna 500 spectrophotometer ranged from 4000 to 400 cm-1.
Thermogravimetric analysis (TGA) was performed in a Mettler Toledo analyzer at a constant heating rate of 10 °C/min under an inert nitrogen flow of 100 mL/min and from room temperature up to 800 °C. The percentage of minerals from the kaolinite group and the ratio dickite/kaolinite were quantified by the Rietveld method and the results were validated with stoichiometric calculations [22] and by thermogravimetric analysis [27].
2.3 Thermal activation
In accordance with TGA and in addition to reduce the power consumption, the excavated clays were calcined at 550 °C and 650 °C and after the calcined excavated clays (AC1-AC5) were also characterized using particle size distribution, specific surface area-Blaine and XRD [13].
2.4 Pozzolanic activity
The calcium hydroxide fixation test was performed on calcined excavated clay / analytical grade Ca(OH)2 pastes that were stored for 14 and 28 curing days. The mix proportion was 1:1 (Ca(OH)2:calcined excavated clay); then 20 mL of distilled water was added, obtaining a relation of solids/water of 1. According to Mendoza and Tobon [28], the percentages of fixated calcium hydroxide in dried pastes were calculated by thermogravimetric measurements (TG) done with a Mettler Toledo in a N2 atmosphere, from room temperature up to 800 °C at a heating rate of 10 °C/min. The TG analysis allowed to identify a mass loss peak around 500 °C, due to the Ca(OH)2 dehydroxylation, and another peak around 800 °C due to the CaCO3 decarbonation.
In order to analyse the effect of the calcined excavated clays in the development of compressive strength of mortars at 7 and 28 days, control mortars were prepared by mixing 1375 g standard Ottawa sand, 500 g Portland cement and water/cement ratio of 0.485, while blended mortars were made by replacing the Portland cement with 20% calcined excavated clays according to previous works [29] and using a water/cementing material ratio necessary for obtain a flow of 110 +/- 5 [30].
2.5 Statistical modelling
The relation among pozzolanic activity of calcined excavated clays with their mineralogical composition, the physical characteristics, the thermal treatments used and the cured time, was studied by different multiple linear regression models. The validity and assumptions of models was based on the determination coefficient (R2), the ANOVA test, the Durbin-Watson statistic and the residual plot.
The R2 measures the goodness of fit of the model and ANOVA test provides the F-value and F-significant to determine whether the model explain a significant quantity of variation. Durbin-Watson statistic is used to identify the existence of linear correlation among independent variables, it ranges from 0 to 4, where a value of 1.5 to 2.5 is considered favourable to obtain suitable models [31]. Residuals are distributed normally when the plot of residuals against the estimate values of dependent variable will show no apparent trend or outliers.
3. Results and discussion
3.1. Characterization of raw excavated waste clays
The chemical compositions of raw materials used are given in Table 1. All the excavated waste clays met the ASTM C 618 requirement for consider them Class N Pozzolans, because of SiO2 + Al2O3 + Fe2O3 is greater than 70 %wt. Cement had an estimated Bogue potential phase composition of 56% C3S, 19% C2S, 7% C3A and 11% C4AF by mass, with gypsum as set regulator. The SO3 content has a marginal influence on the performance of blended cements elaborated with calcined kaolinite clays [32].
From the particle size distribution were calculated d10, d50 and d90 diameters of excavated clays. The d50 of excavated clays was between 8.55 and 19.05 Lim, while specific surface areas Blaine (SSA) were between 4670 and 8769 cm2/g, such as is detailed in our previous paper [13]. Portland cement d10, d50 and d90 was 2.7, 18.3 and 58.6 Lim, respectively.
Source: own elaboration.
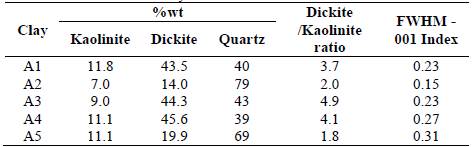
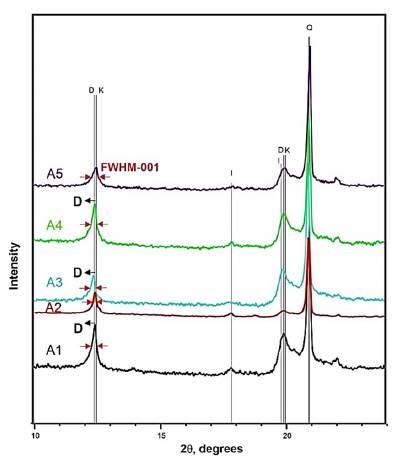
Figure 1 XRD diffractogram of A1-A5 clays. K - kaolinite, D - dickite, I -illite, Q - quartz.
The mineral contents reported in Table 2 were validated by: Rietveld quantitative X-ray diffraction, TGA [27] and stoichiometric calculations [22]. The A1-A5 clays with kaolinite and dickite can be considered as ordered; since these have FWHM-001 index values less 0.3 [26]. The three methods used differ in the percentages obtained by 3%. The other minerals present are identified in Fig. 1. Amorphous phase is obtained after calcination.
The XRD patterns for bulk raw clays are shown in Fig. 1. The main clay minerals identified are kaolinite and dickite (both polymorphs of kaolinite group) and illite with peaks of less intensity. The samples present very strong reflections of quartz, well-defined main reflection of kaolinite (2θ = 12.398°) and dickite (2θ= 12.349°), and a weak main reflection of illite.
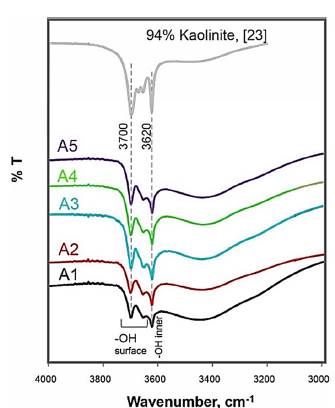
The FTIR spectrums of excavated clays appear in Fig 2. In these spectrums two polymorphs of kaolinite group are identified: kaolinite and dickite, that can be distinguished by differences in position and relative intensity of their OH-stretching bands. The kaolinite spectrum has a strong band at 3700 cm-1 and two weak absorptions at 3669 and 3653 cm-1 [22]. For dickite, the hydrogen bonding between the layers is different: the vibrations of two surface OH, forming stronger hydrogen bonds with the adjacent layers, are coupled and give a strong band at 3653 cm-1 and the third surface OH form weak hydrogen bonds and absorb at 3700 cm-1. The band near 3700 cm-1 of the excavated clays with kaolinite and dickite, has a lower intensity than expected if it only had kaolinite [22,33] and a higher intensity than expected if it only had dickite [33]. The typical strong band at 3653 of dickite does not allow appreciating the typical double at 3669 and 3653 of kaolinite with ordered structure.
3.2. Characterization of calcined excavated waste clays
Thermal treatments modified the mean particle size, the SSA and mineralogy of raw excavated waste clays (A1-A5). Firstly, calcination at 550 °C led to the increase of d50 accompanied by a decrease in SSA for A1 clay, while for A2 and A3 clays led to the increase of both d50 and SSA (Fig.3.).
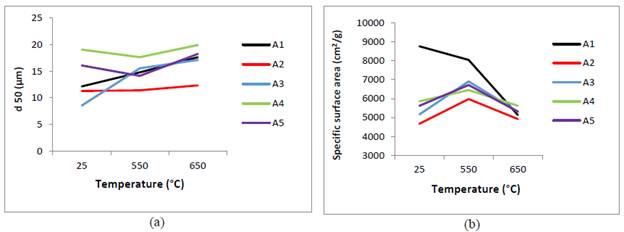
Source: own elaboration
Figure 3 Physical characteristics of excavated wasted clays: (a) d50 (b) Specific surface area.
This apparent contradiction can be explained by considering that the dehydroxylated particles tend to aggregate but producing new porous grains with larger surface [34]. The effect contraction prevailed on the aggregation effect in the A4 and A5 clays given the reduction of d50. It was found that at 650 °C is generated a reduction of specific surface area of A1, A4 and A5 clays, due to increase of d50.
On the other hand, in our previous paper [13] the XRD diagram for excavated clays calcined at 550 and 650 °C indicated that the dehydroxylation take place, since that the corresponding reflections to kaolinite disappeared when A1, A3, A4 and A5 were calcined at 550 °C and when all clays were calcined at 650 °C, while other phases such as illite and quartz remained stable. A2 clay has the highest order, for this reason, the calcination at 550 °C not produces the complete dehydroxylation, and very low intensity peak of kaolinite was identify at this temperature [22].
In addition, in the FTIR spectrums the OH-stretching bands assigned to two polymorphs of kaolinite group are not identified in calcined clays. Both observations confirm the dehydroxylation of the kaolinite group minerals and the formation of a reactive amorphous phase, causing the pozzolanic activity of the calcined clays.
3.3. Pozzolanic activity of calcined excavated waste clays
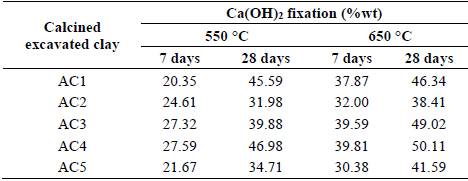
The calcium hydroxide fixation percentages of all blended pastes with excavated clays calcined (AC) at 650 °C were higher than those calcined at 550 °C, at both 7 and 28 days of hydration (Table 3). Which shows a gain in reactivity of calcined clays between these two temperatures. As a result of this, blended cements with excavated clays calcined at 650 °C formed more amounts of C-S-H, C-A-H and C-A-S-H phases, according to the combined water percentages, such as is detailed in our previous paper [13].
The AC4 and AC3 clays showed the highest fixation percentages at 7 days when were calcined either at 550 °C or at 650 °C, while the AC2 and AC5 had the lowest fixation percentages (Fig.4a). The kaolinite and dickite content would explain the pozzolanic activity of these clays, as the higher kaolinite group minerals content in the raw clay, the higher calcium hydroxide fixation; however, when are included other clays that correlation is not good enough, based in R2 value (Fig.4c).
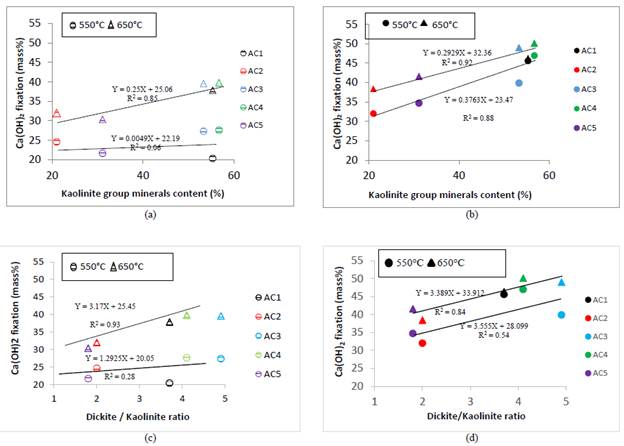
Source: own elaboration
Figure 4 Relationship between CH fixation and kaolinite group minerals content - dickite/kaolinite ratio: (a) - (c) 7 days (b) - (d) 28 days.
Although the A1 clay has a large kaolinite group minerals content, the active phases generated for its calcination at 550 °C just reaction at later ages, and thus, R2 values indicate good correlation with the calcium hydroxide fixation at 28 days (Fig. 4b).
On the other hand, dickite contribute to the pozzolanic activity as the higher dickite/kaolinite ratio in the raw clay, the higher calcium hydroxide fixation (Fig. 4d).
The pozzolanic activity includes both the maximum amount of lime that a pozzolan can combine and the rate at which such combination occurs [35]. The former depends on the nature, quality and quantity of the active phases present in the calcined clays and the later depends on the specific surface area. For this reason, when the rate of pozzolanic reaction varies widely, an extremely low correlation exists between fixed calcium hydroxide percentages at 7 days by excavated clays calcined at 550 °C and kaolinite group minerals content.
The relation between calcium hydroxide fixation percentages and SSA of calcined excavated clays is presented in Fig.5. The correlations are also low, indicating that the pozzolanic activity must be explained by multiple factors in addition to the raw excavated clays kaolinite group minerals content and dickite/kaolinite ratio, for instance by calcination temperature and SSA of calcined excavated clays, such as is proposed in the statistical modelling section.
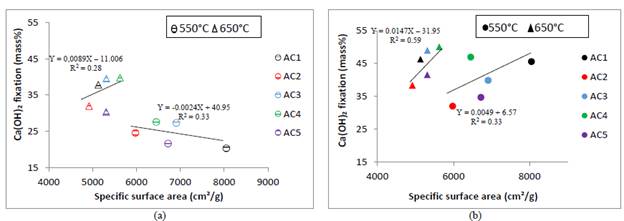
Source: own elaboration.
Figure 5 Relationship between CH fixation and SSA Blaine: (a) 7 days (b) 28 days.
The compressive strength of mortars of Portland cement blended with 20% of excavated clays calcined both at 550 and at 650 °C, curing for 7 and 28 days is presented in our previous paper [13]. The compressive strength development in blended cement with calcined clays depends on PC hydration and the pozzolanic activity of metakaolin obtained from calcination processes ([12], [22]). Pozzolanic reactivity in turn depends on the type and quantity of clay minerals, impurities, thermal activation conditions (temperature, residence time), amorphous phase content, particle size and specific surface area after calcination [2].
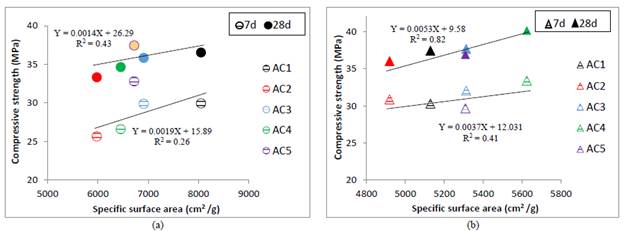
In this sense, the relations between compressive strength and kaolinite group minerals content and dickite/kaolinite ratio are shown in Fig.6. ((a-c) 550 °C and (b-d) 650 °C). At 7 and 28 days, the AC5 clay calcined at 550 °C (Fig.6.a) despite its low kaolinite group minerals content had the highest strength values while AC1 and AC3 clays with medium kaolinite contents had medium strength values. AC2 and AC4 clays had the lowest strength values despite their quite different kaolinite group minerals content.
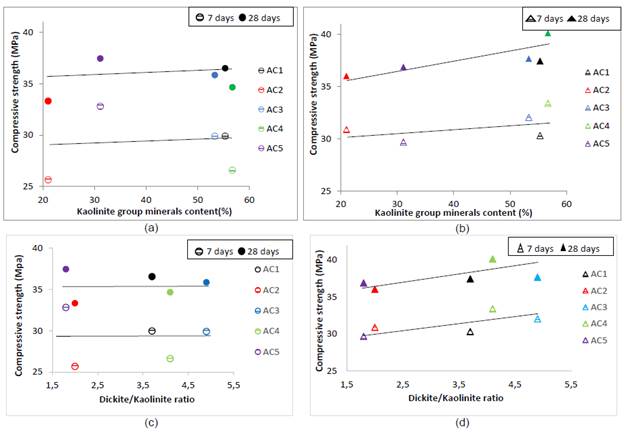
Source: own elaboration.
Figure 6 Relationship between compressive strength and kaolinite group minerals content - dickite/kaolinite ratio: (a) - (c) 550 °C (b) - (d) 650 °C.
Regarding the excavated clays calcined at 650 °C at 7 days, the AC1, AC2 and AC5 clays had the lowest strength values despite high kaolinite group minerals content of AC1 (Fig. 6b).
The AC1, AC3 and AC4 clays has a medium content of kaolinite group minerals, but its structure is classified as ordered (FWHM-001 between 0.23 and 0.2V) causing an active phase with low SSA after thermal treatment that reduces the rate of reaction. The relation between compressive strength and SSA Blaine is presented in Fig.V. ((a) oC and (b) 630 oQ.
After 28 days the strength depends more on the SSA, it is noted that the higher specific surface area, the higher compressive strength; even the AC4 clay with the highest SSA developed the same strength that mortar control. The low correlations indicate that the pozzolanic activity must be explained by multiple factors in addition to the raw excavated clays kaolinite group minerals content, for instance by dickite/kaolinite ratio, calcination temperature and SSA of calcined excavated clays.
3.4. Statistical modelling
To study the thermal treatment effect on the calcium hydroxide fixation and the compressive strength of samples blended with calcined clays, in our previous paper [14] was established an experiment design of two factors in randomized complete blocks. Each kind of excavated clay was considered as a block and the two factors were the calcination temperature and the hydration age.
In this work, different models were proposed to find and to quantify the best relation among fixed calcium hydroxide (CHFt) by calcined excavated clays and the mineralogical composition, the physical characteristics and the thermal treatments, under experimental domain of interest for each variable. The model that better fitted the relationship between response and independent variables was a linear combination of kaolinite content (K), dickite/kaolinite ratio (DK), the levels coded for low and high values of temperature of calcination (T) and the hydration age (H), under experimental domain of interest for each variable and as the regression equation:
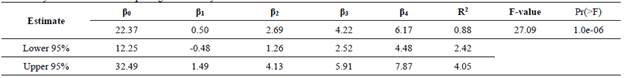
The calcination temperature is considered as a control factor because can be controlled for instance, during industrial production of calcined clays. The regression coefficients (Pi) estimated by least squared method with statistical software R, are related in the Table 4. Coefficient determination R2 indicates that the independent variables explain up to 88% the total variation of calcium hydroxide fixation. Results of ANOVA, show that F-value is greater than value obtained for a distribution Fa,k,n-k-1, with a=0,01, k= 4 variables, n= 15 residuals. Considering 99.9% confidence level, at least one independent variable contribute significantly to the regression as the p-value was lower than 0.001.
The regression model assumptions are met, Durbin Watson Statistic was of 1.84; then the model is unaltered by multi collinearity. The plot of the standardized residuals versus the predicted values of calcium hydroxide fixation showed a random distribution, validating the model adequacy (Fig.8 (a)). There were not outliers, but the model can be less accurate to estimate fixations around 28%, due to the higher residuals.
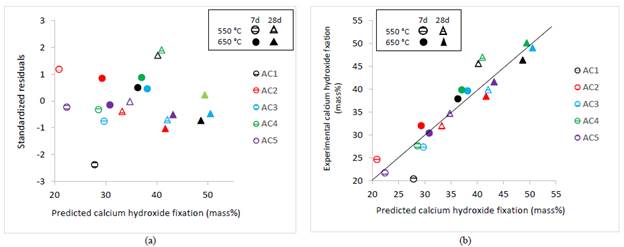
Source: own elaboration
Figure 8 Predicted calcium hydroxide fixation and: (a) Standardized residuals (b) Experimental calcium hydroxide fixation.
The relationship between the calcium hydroxide fixation predicted by multiple regression analysis and the experimental values (Fig. 8 (b)) show the goodness of fit of the regression model. Coefficients estimated are useful for comparing the relative influence of kaolinite, dickite/kaolinite ratio, the calcination temperature, and the hydration age (Table 4); under experimental domain of interest for each variable. The higher kaolinite content and DK ratio the higher reactive silica and alumina fractions generated by the thermal treatment, which will fix more calcium hydroxide.
The model states that by increasing kaolinite from 7 up to 11.7%, the calcium hydroxide fixation will be increased around 12%, while it will be increased around 41% when dickite/kaolinite ratio moves from 1.8 up to 4.9 (Table 2). Also, by increasing T from 550 to 650 °C, it will be increased around 41%; while a variation of H from 7 to 28 days will produce a variation of around 60%.
Likewise, was modelled by different regression models the relation among the compressive strength of calcined excavated clays and different characteristics. The model better fitted was a linear combination of kaolinite, dickite/kaolinite ratio, the SSA of calcined excavated clays, the levels coded for low and high values of temperature of calcination (T) and the curing time (C), as the regression equation:
The regression coefficients (βi) estimated by least squared method with statistical software R, are related in the Table 5. Coefficient determination R2 indicates that the independent variables explain up to 86% the total variation of compressive strength.
Results of ANOVA, show that F-value is greater than value obtained for a distribution Fα,k,n-k-1, with α=0,01, k= 5 variables, n= 14 residuals. Considering 99.9% confidence level, at least one independent variable contribute significantly to the regression as the p-value was lower than 0.001. The regression model assumptions are met, Durbin Watson Statistic for regression model was of 1.9, therefore the variables are independent.
The plot of the standardized residuals versus the predicted values of compressive strength showed a random distribution, validating the model adequacy (Fig.9 (a)).
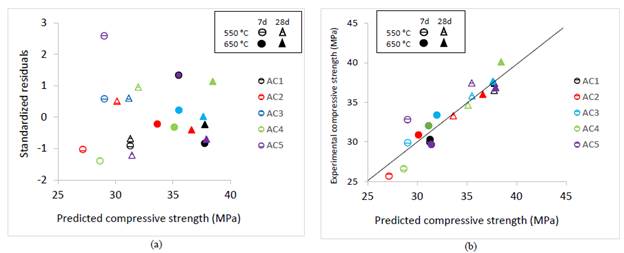
Source: own elaboration
Figure 9 Predicted CS and: (a) Standardized residuals (b) Experimental compressive strength.
The relationship between the compressive strength predicted by multiple regression analysis and the experimental values (Fig. 9 (b)) show the goodness of fit of the regression model.
Coefficients estimated are useful for comparing the relative influence of kaolinite, dickite/kaolinite ratio, the SSA, the calcination temperature, and the curing time, under experimental domain of interest for each variable. The higher kaolinite content and dickite/kaolinite ratio (1.8 - 4.9) the higher reactive silica and alumina fractions, generated by the thermal treatment and associated with increased lime fixation, which will contribute to improve the compressive strength. As SSA provides active nucleation sites, it contributes to enhance early strength by accelerated hydration of cement.
The model states that the compressive strength at 28 days increased 5%, when the kaolinite increased up to 20% and dickite up to 40% for a clay with 10% of kaolinite, 20% of dickite, SSA of 4000 cm2/g and calcined at 550 °C. Also, by increasing SSA up to 6000 cm2/g and calcination temperature up to 650 °C, the compressive strength increased 10% and 15%, respectively. So, the total increase was 30% when the three variables were increased together.
4. Final discussion
The analysis of the experimental results obtained throughout this investigation clearly show that excavated waste clays, even with low kaolinite content, if they are properly thermal treated, can become good supplementary cementitious materials with important lime fixation values and significant contributions to the development of compressive strength. For example, samples with a low kaolinite group minerals content (20 to 30%), when calcined at 650 °C, fix around 40% of the available lime and when they are added 20% in mortars, they reach compressive strength values close to 90% of that obtained with the control sample. With medium kaolinite group minerals content (50%) fix around 50% of the available lime and in mortars they reach compressive strength close to 100% of those values obtained with the control sample. This showed that although there is a tendency to have better lime fixation and compressive strength results with increase of kaolinite group minerals in the samples, the correlations found among these parameters using linear regressions were not good. That is to say, the low correlations observed indicate that the pozzolanic activity will depend on multiple mineralogical-structural, physical and processing characteristics of the clays, such as dickite/kaolinite ratio, specific surface area and thermic treatments, among other.
Therefore, it is being proposed in this paper the use of multiple regressions model for pozzolanic activity prediction of excavated waste clays with low and medium kaolinite group minerals content.
5. Conclusions
The excavated waste clays can develop pozzolanic activity by thermal activation at 550 and 650 °C. According to calcium hydroxide fixation test, raw excavated clays with kaolinite group minerals contents between 20 - 60% are reactive after their calcination.
The multiple regression models demonstrate that the compressive strength depends on the kaolinite content, dickite/kaolinite ratio, specific surface area Blaine, calcination temperature and curing time, and the contribution of these factors on compressive strength is time dependent.
It is possible to increase the calcium hydroxide fixation of calcined excavated clays by increasing the calcination temperature. The compressive strength of blended mortar is high at temperature calcination up to 650 °C, and in these conditions, the compressive strength increasing with increasing the specific surface area Blaine; from a technological standpoint, the modification of any of those variables can improve the compressive strength.
The proposed models are statistically sufficient because satisfy the ANOVA test, the Durbin-Watson statistic, and the residual plot. The determination coefficient indicates that the linear model is a good description for the relationship among the variables.