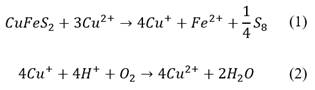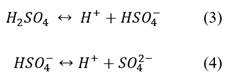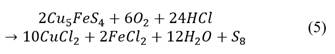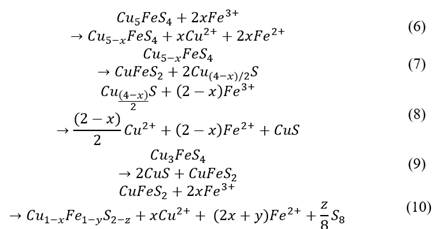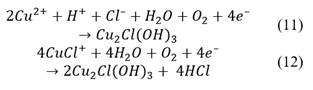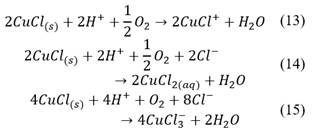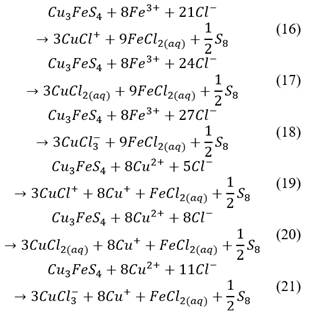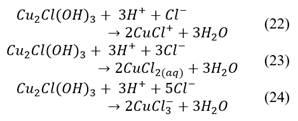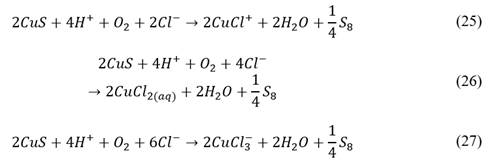1. Introduction
Bornite (Cu5FeS4) as a copper rich mineral and as such a natural source of copper can be frequently found in equilibrium with the two most common copper sulfur minerals, chalcopyrite (CuFeS2) and chalcocite (Cu2S) [1,2]. Furthermore, these minerals are well known for being refractory ores, i. e., materials that pose challenges considering the dissolution in leaching solutions [3]. For this reason, studying the leaching behavior of bornite presents a significant economic relevance [4].
Due to the depletion of copper oxide ores in the past few years, the presence of toxic elements in concentrates such as arsenic (which is carcinogenic to humans) and due to the increasing relevance of ESG (environmental, social & governance) regulation risk management, the hydrometallurgical option for processing flotation concentrates has become more attractive, because it represents an environmentally friendlier process compared to pyrometallurgical processes [5,6]. In this aspect, research for new leaching methods touching on copper sulfur ores using hydrometallurgical techniques, has been intensified [7-11].
Several studies on the leaching of bornite have been carried out [12-16], but none of them had achieved the goal of extracting all the copper from bornite at standard temperature and pressure conditions.
In the chalcopyrite leaching field, historically some authors have applied pre-treatments to sulfide copper ores before the leaching, where the pre-treatment with H2SO4 and NaCl had reported promising results [17-21]. Pre-treatment is a necessary step to improve the leaching of copper oxide and secondary sulfide ores. Acid pre-treatment of ores using H2SO4 produces dehydration and inhibits gangue dissolution, making an increase of the copper leaching rate by the formation of soluble sulfur-copper complexes. Also, it passivates the well-known acid-consuming gangue [22].
Leaching with sulfate/ferric chloride, a complex process, has not been mechanistically elucidated. However, the dissolution of bornite in the H2SO4/Fe3+ medium can be divided into two stages. In the first one, preferential removal of copper leads to the formation of a non-stoichiometric bornite (Cu5-xFeS4), which is progressively transformed into chalcopyrite and non-stoichiometric chalcocite (Cu2-xS). At low temperatures, this stage ends when an idaite (Cu3FeS4) composition is reached (2CuS·CuFeS2). In the second stage, sulfide from idaite phase is oxidized. At the same time the oxidations state of the metals remains unchanged, which is a reaction that only occurs by leaching at higher temperatures [4,14,23-29].
The oxidation states of copper in bornite, chalcopyrite and covellite have been extensively studied, but not well understood yet, being probably a mixture of Cu(I) and Cu(II) [30]. The copper - over the iron - is preferentially extracted from the bornite (resulting in a Cu5-xFeS4 composition) since the iron atom is well fixed in the crystal lattice of bornite [31]. At the same time, the Cu+ can diffuse more freely, resulting in a faster extraction [32].
The addition of Fe(III) during the leaching stage of bornite is not necessary, as demonstrated by Navarro et al. [21] since the same ore carries soluble iron species. When Fe(II) is in solution, it can be oxidized to Fe(III) by the oxygen present in the medium [9]. Thus, Fe(III), acting as an oxidant, improves the leaching of bornite while adding more Fe(II) to the solution, which can be re-oxidized to Fe(III).
The characteristics of the chloride ion (Cl-) in the leaching of sulfide minerals have been thoroughly studied, finding that the leaching speed in acid-chloride solutions is faster than those without chloride ions [33-35], due to the increase of porosity on the surface of the ores, which helps to overcome the passivation of minerals [9, 36]. It is also well known that the presence of chloride stabilizes Cu(I) ions.
This property can enhance the leaching of some copper ores (e. g. chalcopyrite) due to the formation of a Cu(II)/Cu(I) couple, where the Cu(II) will act as an oxidant [36]. Corresponding mechanism is presented in eq. (1)-(2), where the copper within the chalcopyrite is assumed to be as Cu(I).
It can also be noted that, in the absence of NaCl in H2SO4 solutions, eq. (4) can be neglected, as Na+ ions would associate with hydrogen sulfate ions, favoring the equilibrium of the reaction in eq. (3) to the right-hand side and thus decreasing the pH of the solution [37].
Nowadays many copper mines use seawater to fabricate the leaching solution, basically due to its abundance and its high chloride content [38,39].
That said, this study aim to determine the effect of HCl on formation of soluble species from a flotation bornite concentrate pre-treated with HCl under different conditions of acid concentration and ore particle size, with a posterior orbital leaching in a H2SO4-NaCl solution, all under standard conditions.
2. Materials and methods
2.1. Materials
Our experimental study uses a bornite concentrate obtained through a froth flotation process. The characteristic particle size of the concentrate was T80 = 72 µm, and its composition was 57.4% Cu, 9.13% Fe, 24.1% S and 0.0730% As. This composition was determined using atomic absorption spectrophotometry (AAS) (GBC Scientific Equipment Spectrophotometer, SensAA, Dandenong Victoria, Australia).
Powder X-ray diffraction (Siemens® D5000 X-ray diffractometer, The Woodlands TX, USA) showed the relative presence of the following mineralogical species: Cu5FeS4, CuFeS2, Cu7S4, Cu3AsS4 and CaCO3.
With this information in hand, we could determine the weight percentage of every specie in the concentrate, which is presented in the Table 1.
2.2. Reagents
Technical grade hydrochloric acid (37.0% pure) was used to the pre-treatment of the concentrate. To simulate the use of seawater and to compare with previous work [21], NaCl (99.9% pure) and H2SO4 (98.0% pure) were used to prepare the leaching solution. Water was deionized before use.
2.3. Methods
2.3.1. Concentrate washing
The concentrate was washed to remove residual reagents carried over from the flotation stage (e.g., surfactants, depressants, frothers, etc.). 1 L of distilled water was used for every 100 g of concentrate. First, water and concentrate were mixed in a reagent flask and stirred at 60 °C for 30 min. After this, the mixture was filtered and then the concentrate was dried at 45 °C for 48 h.
2.3.2. Particle separation
Once the concentrate was washed and dried, it was separated into four particle sizes, using a Ro-Tap and four ASTM sieve sizes: #100, #200, #270 and #400. The particle size ranges obtained are shown in Table 2.
2.3.3. Pre-treatment
The pre-treatment consisted of adding three different doses of technical hydrochloric acid (37.0% pure) to 3 g samples of washed concentrate previously moisturized with distilled water in Petri dishes. These dosages corresponded to 5, 10 and 15% of the stoichiometric consumption of HCl by the bornite, considering the complete dissolution of bornite by the hydrochloric acid under the presence of oxygen, as showed in eq. (5). The amount of water added was calculated to reach an overall humidity of 20%.
After this, the samples were stored in an oven at 25 °C for 15 d, adding distilled water periodically to compensate gradual evaporation.
Every sample was doubly prepared to determine its mineralogical composition with powder XRD (X-Ray Diffraction) techniques once the pre-treatment was done. In other words, 24 samples (four particle sizes and three acid dosages, with their respective duplicates) were pre-treated at 25 °C for 15 d, where half were leached, and the other half were ground and stored for a posterior analysis by powder XRD. Also, samples of concentrate without pre-treatment for each particle size were leached and the leaching residues were analyzed by powder XRD and SEM (Scanning Electron Microscopy). In sum, a total of 28 samples were used in this research.
2.3.4. Leaching
Once the pre-treatment was completed, each sample was orbital leached at 180 rpm, in a 500 mL closed cylindrical reactor for 24 h at 25 °C. 300 mL of leaching solution ([Cl-] = 60 g·L-1, pH = 1.5) were used per sample, using NaCl and H2SO4. Furthermore, control samples of concentrate with no pre-treatment for each particle size studied were leached under similar conditions. The oxidation-reduction potential (ORP) and the pH of the solution were measured with a pH/ORP meter (Hanna portable pH/ORP meter, model HI 2002) with a silver/silver chloride reference electrode while the leaching was carried out. The pH was adjusted during the process, adding sulfuric acid when it was necessary. Aliquots of 3 mL were taken at 1, 2 and 24 h, where Fe2+ and copper concentrations were measured by AAS.
The solutions were filtered when the leaching stage was completed, and the liquid phases were separated from the solid residues. These residues were then washed with distilled water and dried for 48 h. After this, they were analyzed with XRD techniques to determine their mineralogical composition.
3. Results and discussion
3.1. Copper stable compounds
Using the Medusa software [38] it was possible to identify stable ion species during pre-treatment. Results showed that, for three HCl concentrations used (see Table 3), the most stable species were CuCl+ and Cu2+. For leaching conditions (see Table 4) the most stable copper species were CuCl+, CuCl2(aq) and CuCl3 -. Cu(I) species were not considered due to their low presence, thus neglected from calculations.
Table 3 Pre-treatment conditions for each dosage studied
| Dosage | H2O (mL) | Cu dissolution* | Cu2+ (M) | Fe dissolution* | Fe2+ (M) | Cl- (M) | pH |
|---|---|---|---|---|---|---|---|
| 5% HCl | 0.75 | 18% | 6.503 | 4.5% | 0.294 | 3.189 | -0.504 |
| 10% HCl | 0.75 | 28% | 10.12 | 3.5% | 0.229 | 6.379 | -0.805 |
| 15% HCl | 0.75 | 37% | 13.37 | 2.5% | 0.163 | 9.568 | -0.981 |
*: Average dissolution reached at 1st hour of leaching
Source: The Authors
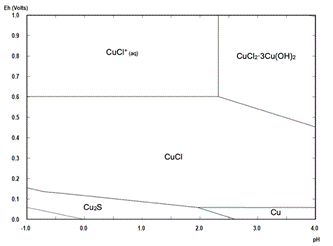
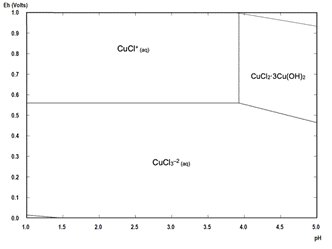
A Pourbaix diagram for the Cu-Fe-S-Cl-H2O system at 25 °C under pre-treatment conditions focused on the copper species is shown in Fig. 1 and focused on the iron species is presented in Fig. 2. These diagrams are valid for the three HCl concentrations studied in this paper and the solution conditions are presented in Table 3. Copper and iron concentrations were considered equal to the average dissolution reached at the first hour of the leaching stage, as it was assumed that this first hour of leaching represents the dissolution of soluble species formed during the pre-treatment. It can be seen from Fig. 2 that the iron in solution stays as Fe3+, acting as an oxidant.
Source: The Authors
Figure 1 Pourbaix diagram for the Cu-Cl-Fe-S-H2O system at 25 °C under pre-treatment conditions used during this study.
Source: The Authors
Figure 2 Pourbaix diagram for the Fe-Cu-Cl-S-H2O system at 25 °C under pre-treatment conditions used during this study.
In the same way, a Pourbaix diagram for the Cu-Fe-S-Cl-H2O system at 25 °C under leaching conditions focused on the copper species is shown in Fig. 3 and focused on the iron species is shown in Fig. 4. Copper and iron concentrations were considered to be equal to the average dissolution reached at half of the leaching time (12 h), all presented in Table 4. Again, it can be seen from Fig. 4 that the iron in solution is preferentially oxidated to Fe3+, acting as a leaching agent.
Source: The Authors
Figure 3 Pourbaix diagram for the Cu-Cl-Fe-S-H2O system at 25 °C under leaching conditions used during this study.
Source: The Authors.
Figure 4 Pourbaix diagram for the Fe-Cu-Cl-S-H2O system at 25 °C under leaching conditions used during this study.
Table 4 Leaching conditions for each dosage studied.
| Dosage | H2O (L) | Cu dissolution* | Cu2+ (M) | Fe dissolution* | Fe2+ (M) | Cl- (M) | Na+ (M) | HSO4 - (M) | pH |
|---|---|---|---|---|---|---|---|---|---|
| 5% HCl | 0.3 | 26% | 0.023 | 8.3% | 0.0013 | 5.650 | 5.642 | 0.024 | 1.5 |
| 10% HCl | 0.3 | 34% | 0.031 | 6.5% | 0.0011 | 5.658 | 5.642 | 0.024 | 1.5 |
| 15% HCl | 0.3 | 43% | 0.039 | 8.8% | 0.0014 | 5.666 | 5.642 | 0.024 | 1.5 |
*: Average dissolution reached at 12th hour of leaching.
Source: The Authors
It is important to note that Pourbaix diagrams display the stable species when equilibrium is reached, to observe differences in experimental processes.
3.2. During pre-treatment observed effects of aqueous HCl solution concentration on the formation of soluble species
3.2.1. Characterization of the pre-treated concentrates
Using SEM and XRD techniques, the mineralogical composition of T80 = 72 µm and between -53+38 µm pre-treated with 5% and 15% HCl were determined.
Table 5 provides information about the mineralogical species obtained at the end of the 15 d of pre-treatment. Each sample contained chalcopyrite and soluble atacamite type compounds. Here, through eq. (6)-(10), a possible mechanism is proposed, in accordance with Dutrizac et al. [15]. Eq. (6) suggests that the first step in the ferric ion leaching of bornite is to transform it into non-stoichiometric bornite and copper ions, where “x” is believed to take values between 0 and 2. Samples pre-treated with 5% HCl showed presence of bornite after 15 d of pre-treatment, while samples pre-treated with 15% HCl did not. This can be explained by looking at eq. (7), This reaction was not completed for samples pre-treated with 5% HCl. In comparison, samples pre-treated with 15% HCl were fully transformed into chalcopyrite and nonstoichiometric chalcocite, the latter further oxidized through eq. (8), probably reaching an idaite composition (CuFeS2•2CuS). This idaite stage probably decomposed into chalcopyrite and covellite, as proposed in eq. (9), which could explain the presence of Cu34S32. At some point, chalcopyrite might be leached as proposed in eq. (10), which would explain the detection of Cu4Fe5S8 (Cu0.8FeS1.6) by the XRD. The values of x, y and z in this equation are not well clarified, but clearly x & y cannot be more significant than one and z bigger than two [41, 42].
Table 5 Effect of aqueous HCl solution concentration during the pre-treatment on the mineralogical composition of the concentrate samples.
| Sample | 5% HCl | 15% HCl |
|---|---|---|
| T80 = 72 µm |
|
|
| -53+38 µm |
|
|
Source: The Authors
Since water in the mixture evaporated as the days passed, it had to be refilled constantly during pre-treatment, which could have produced pH variations, leading to an atacamite formation (see Fig. 1), as proposed in eq. (11)-(12).
The presence of nantokite (CuCl) can be related to possible potential variations of the mixture during the pre-treatment resting time, where if the potential goes under 0.6 V versus SHE, the Pourbaix diagram presented in Fig. 1 predicts its formation. However, as the potential was between 0.4-0.7 V versus SHE during leaching, this copper salt does not represent a problem because it can be leached as shown in Figure 2 and eq. (13)-(15).
3.2.2. Leaching results
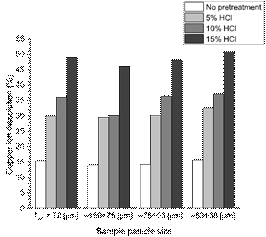
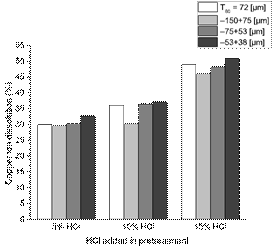
As displayed in Fig. 5, the observed tendencies regarding the copper ion dissolution, for all four particle sizes analyzed, were similar. Increasing the concentration of HCl in an aqueous solution during pre-treatment led to an improvement in dissolution after 24 h of leaching. It is important to highlight that the copper ion recovery from the samples without pre-treatment were the lowest among all the samples.
3.2.3. Characterization of the leaching residues
Table 6 shows the mineralogical composition of leaching residues. The samples pre-treated with less concentrated HCl solution contained non-stoichiometric bornite. This means that these samples did not have sufficient acid during the pre-treatment to complete the transformations to soluble species, which led to a less efficient copper dissolution during the posterior leaching stage. The main reaction for samples pre-treated with 5% HCl is represented by eq. (6) with “x” taking values probably between 0 and 2.
Table 6 Mineralogical composition of the leaching residues
| Sample | 5% HCl | 15% HCl |
|---|---|---|
| T80 = 72 µm |
|
|
| -53+38 µm |
|
|
Source: The Authors
On the other hand, bornite of samples pre-treated with 15% HCl was completely transformed into idaite during pre-treatment, therefore, in the leaching stage, sulfide present in idaite could have been oxidized by Fe(III) and Cu(II), which both get reduced, as proposed in eq. (16)-(21).
The atacamite formed during pre-treatment for all HCl concentrations used must have been almost completely transformed into copper ions and water, as proposed in eq. (22)-(24), which could explain why it did not appear in Table 6.
Covellite is a well-known refractory ore, and it cannot be oxidized by Fe(III). When covellite is in the medium (due to idaite decomposition), it is slowly leached by oxygen in solution as presented in eq. (25)-(27). Thus, a longer leaching time would be necessary to leach all the covellite.
Finally, as proposed in eq (10), part of the chalcopyrite might have been leached, more likely on samples pre-treated with higher HCl.
Comparing Fig. 6 with Fig. 7 below, one can see that the first one shows no visible effects of leaching on the particles, while in the latter there are a lot of smooth cracks on the surface of the particles. These fissures are deeper in the sample pre-treated with 15% HCl solution, which here corresponds to Fig. 8, where some micro pores surrounding the fractures are clearly visible.

Source: The Authors
Figure 6 SEM image from the leaching residue of the -53+38 µm particle size concentrate without pre-treatment.

Source: The Authors
Figure 7 SEM image from the leaching residue of the -53+38 µm particle size concentrate pre-treated with 5% of HCl.

Source: The Authors
Figure 8 SEM image from the leaching residue of the -53+38 µm particle size concentrate pre-treated with 15% of HCl.
Putting these results together with the leaching results from Fig. 5, it is feasible to relate the presence of fissures and pores to the copper dissolution. In fact, one can assume that these observations are due to the pre-treatment stage effect of the HCl, as for the sample without pre-treatment there was no evidence of cracks or micro pores detected by electron microscopy, hence making this stage of treatment crucial for the copper recovery. Thus, it can be said that the direct contact of an HCl solution with bornite particles is responsible for the fractures and pores due to the HCl highly corrosive properties. Carneiro & Leão [36] stated in their paper that Cl- ions play a crucial role in the changes observed on the surface form and properties of the reaction product during the acid leaching of chalcopyrite, where “…in the presence of NaCl, surface area and porosity of the leach residue are higher than those obtained without sodium chloride”.
The formation of fissures and pores is beneficial for copper dissolution, increasing the internal surface of particles, allowing for deeper penetration of HCl solution within each particle and avoiding a further passivation of ore metals by forming air-oxidation stable complexes at the same time.
3.3. During pre-treatment observed effects of particle size on the formation of soluble species
3.3.1. Characterization of the pre-treated concentrates
Considering the results presented in Table 5, in view of the particle size, it is evident that the composition between samples with the same particle size are almost identical.
3.3.2. Leaching results
Fig. 9 illustrates the copper dissolution vs. HCl dosage during pre-treatment stage, organized by particle size. For each dosage of HCl, the copper recovery was similar. This means that the particle size does not have an important role on the formation of soluble species, at least in the size range evaluated in this paper. As discussed earlier, liquid HCl during pre-treatment led to the presence of fissures and micro- the difference in particle size negligible for the size range studied. These results corroborate the results presented in Table 6.
3.3.3. Characterization of the leaching residues
Considering the results presented in Table 6, because of the particle size one can recognize that all ore samples pre-treated with 15% HCl led to almost the same composition. The only noticeable difference corresponds to the presence of CuCl.
If one compares Fig. 7 with Fig. 10, it is possible to observe an identical effect over the surface of the particles, caused by the HCl, which induced cracks in them. The same comparison can be made for Fig. 8 with Fig. 11, where the width and depth of the fractures were clearly amplified by the addition of more concentrated HCl, with a noticeable effect on the copper ion recovery, as evident from Fig. 9. These hints to why particle size is a less relevant factor, as bigger particles were as fractured as smaller ones, solving the problem of possible passivation, and allowing for an efficient chemical dissolution of the relevant metal species from within.
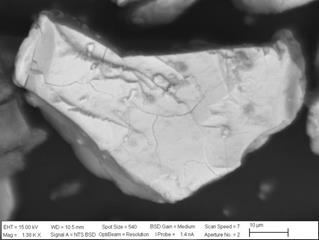
Source: The Authors
Figure 10 SEM images from the leaching residue of the T80 = 72 µm particle size concentrate pre-treated with 5% of HCl
4. Conclusions
By the experimental results obtained in this study, in which the concentration of HCl added and particle size on the formation of soluble species during the pre-treatment of a flotation bornite concentrate were studied, it was possible to improve the copper dissolution from 13.0% to 49.0%, pre-treating the concentrate for 15 days at 25 °C with 15% of the stoichiometric quantity of HCl needed to fully dissolve the bornite, without modifying its initial particle size. Also, the soluble copper species formation of the bornite during the pre-treatment with HCl was not affected by the particle size of the samples. This was deduced from the SEM images obtained from the leaching residues, where we could see that the HCl was capable of completely fracturing the big particles as well as the small ones, avoiding the passivation and inducing changes from within.
Increasing the HCl pre-treatment concentration considerably improved the final Cu recovery, where we identified a crucial dosage of 15% for which the bornite was transformed mainly into non-stoichiometric bornite, chalcopyrite and covellite. We believe that better results can be obtained if the pre-treatment is carried out with a higher initial liquid HCl concentration, potentially reaching recoveries high enough to have an economically feasible high-scale process. Nevertheless, more research is required.