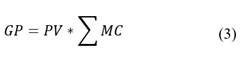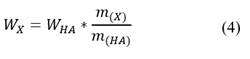1. Introduction
At present, one of the major environmental problems is the impact of the intensive and inadequate use of chemical fertilizers, what causes soil degradation and loss of its productive capacity, the contamination of food for human consumption and water pollution [1].
Mineral fertilizers have a negative impact on the physical properties and fertility of the soil, by the doses and forms of application. The accumulation of pollutants in soils poses a risk for the immediate and long-term future, since the progressive increase of such contaminants can transform them into unstable systems (ecosystem degradation) and increase agricultural production costs [2]. In addition, contaminated or poorly treated soils may also affect the water and the atmosphere and generate harmful effects for humans, wildlife in general and vegetation.
A solution has been the use of organic fertilizers, where the humic substances (HS) play a decisive role, due to the capacity of absorption and mobilization of nutrients by the plants, so that each biosynthesis process is optimized with productive and qualitative benefits [3,4]. So far, HS have been used mainly to improve soil fertility conditions and optimize the structure, permeability, and levels of organic matter in soils, although there are foliar application studies [5].
HS are defined as dark-colored amorphous polymers, which are synthesized biochemically and/or chemically in the environment from biomass constituents or their metabolites [6]. HS are classified in function on their solubility and are divided into three groups. Humic acids (HA) are the fraction of HS that are insoluble in water under acidic conditions (pH <2), but soluble at higher pH values. However, fulvic acids are soluble in water throughout the pH range, while humin represents the insoluble fraction at any pH value. It is often referred to as the highest molecular weight fraction (1500-5000Da and 50,000-500,000Da in streams and sediments, respectively. On the other hand, fulvic acid (FA) is soluble in water at any pH conditions, it contains more acidic functional groups and it ranges from 600to 1000 Da in streams and a little bit higher in soils (1000 to 5000 Da). The humin molecular weight is considerably higher than the rest.
Due to their deposition tendencies, ground accumulation and sedimentation, these molecules became the main kerogen, a complex fossilized organic material found in oil shale and other sedimentary rocks. This material is insoluble in common organic solvents and yields petroleum products on distillation. The bulk of organic matter in sedimentary rocks appears in the form of kerogen: complex polymeric material that is insoluble in inorganic solvents, and difficult to analyze chemically precursor [7].
HS are one of the main classes of organic matter and contain carboxylic, phenolic, and hydroxyl groups. The presence of these groups makes HS attractive for environmental remediation, because it is by means of these groups that the HS can bind to organic and inorganic pollutants, what leads to the decrease of the toxicity and the increase of the cation exchange capacity (CEC) of the soil [8].
The differences between humic and fulvic acids from the point of view of their applications are mainly related to their molecular weight and their mobility in soil solution. Thus, HA with high molecular weight have a greater impact on the physical properties and local biological effects of the soil, whereas low molecular weight FA influences in the transport of micronutrients in soil solution. In addition, they also exert biological effects in the rhizosphere [9].
The role of HA in agricultural soils is well established, especially in those soils with low organic matter, and also too in pollution remediation [9]. HA has long been used in improvement crop productivity and soil fertility. Authors various [10] relate numerous studies that show the beneficial effects of HS on specific crops, such as barley, olive trees, corn, oats, grapes, cocoa trees and tomatoes.
In medicine, HA is has several uses: as antiviral and anti-inflammatory agent, in wound healing, cancer and prion disease therapy and as antimutagenic/desmutagenic potential. In the pharmaceutical and cosmetic areas, they are known to protect against UV-VIS radiation and to act as antioxidants. Further applications include their use as solubilizing agents and for transporting hydrophobic active compounds, two applications that may be improved by their administration as HA-surfactant nanoparticles [11].
There is a great number of methods for extraction of HS, with different extraction reagents [8,12-15]:
The strong Reagents (NaOH - KOH) are commonly used for the isolation of HS present in organic matter. Extraction of HS from organic materials with alkali solution leads to the recovery of approximately two-thirds of the organic matter. The amount of organic matter extracted with caustic alkali increases with time of extraction.
The main mild reagents is Na4P2O7, although, there are other neutral salts. As note earlier, the amount of organic matter recovered (<30%) is considerably less than when use caustic alkali, but less alteration occurs. To minimize chemical modification of the humic material, extraction should be carried out at pH 7.0. This method recommends some alternatives with strong alkali to increase extraction.
The use of organic chelating agents (acetylacetone, cupferron and hydroxyquinoline) is one way to increase effectiveness by mixing these reagents with other products such as urea at high concentrations.
A method with formic acid (HCOOH) has been used to remove HA present in soil and compost.
The objective of this paper is to evaluate the extraction of HS (HA and FA) from three organic materials, with different basic and acid extractants.
2. Materials and methods
2.1. Preparation of raw materials used
Three types of raw material are used: vermicompost, compost and garden waste. Vermicompost and compost were obtained from the Provincial Soil Laboratory in Matanzas. Garden waste comes from the green areas of the University of Matanzas. Raw materials were sampled at random and passed through a 2 mm sieve [15]. The garden waste sample was ground before application.
The three materials are characterized. Humidity, organic matter, phosphorus, potassium, sodium, calcium and magnesium content are determinate.
2.2. Methodologies of extraction of humic substances
Two methods are used for the extraction of humic substances [8,13]. Both are based on two fundamental stages: basic extraction with NaOH (0.1 mol.L-1) or KOH (0.1 mol.L-1) respectively as basic extractants (BE), and acid extraction with HCl (6 mol.L-1) or H2SO4 (6 mol.L-1) as acid extractants (AE), respectively (see Table 1).
Table 1 Description of methods used in the work.
| Method | Basic Extractant | Acid Extractant |
|---|---|---|
| I | NaOH | HCl |
| II | KOH | H2SO4 |
Source: Own elaboration
The first stage is the separation of HS. The sample is weighed and then the basic extractant is added with raw material/extractant ratio of 1/10 [15]. It is stirred continuously for 12 hours and centrifuged. The supernatant is the HS and the precipitate correspond to the humin. The raw materials sample mass was 0.5 g for all experiments.
The second stage is the fractionation of HS into HA and FA. The HS are acidified with HCl or H2SO4 to a pH value between 1 and 2, they are left to stand for 24 hours and centrifuged. The supernatant is the FA and the precipitate is the HA.
A pH meter model PHSSJ-3F is used to measure the pH, and the density from a Gay Lussac 25 mL pycnometer.
2.3. Experimental design
In this investigation a Multi-Level Factorial experimental design is planned. The factors to be studied are: 1) type of basic extractant (NaOH or KOH), 2) type of acid extractant (HCl or H2SO4) and 3) type of raw material (RM): vermicompost (VC), compost (C) and garden waste (GW). Table 2 shows the alternatives proposed in the study.
Table 2 Experimental field definition for the Multi-Level Factorial design.
| Alternative | Raw materials | Basic Extractant | Acid Extractant |
|---|---|---|---|
| 1 | Garden waste | NaOH | H2SO4 |
| 2 | Garden waste | KOH | HCl |
| 3 | Vermicompost | KOH | H2SO4 |
| 4 | Compost | KOH | HCl |
| 5 | Garden waste | KOH | H2SO4 |
| 6 | Garden waste | NaOH | HCl |
| 7 | Vermicompost | NaOH | H2SO4 |
| 8 | Vermicompost | NaOH | HCl |
| 9 | Vermicompost | KOH | HCl |
| 10 | Compost | KOH | H2SO4 |
| 11 | Compost | NaOH | H2SO4 |
| 12 | Compost | NaOH | HCl |
Source: Own elaboration
The variables responses determined in this research are:
Volume of humic substances: V(HS). (the supernatant of the basic extraction, for gravimetric methods).
Mass of dried Humin: m(H), g (the precipitate of the basic extraction, for gravimetric methods).
Volume of acid extractant: V(AE), mL.
Volume of FA: V(FA), mL (the supernatant of the acid extraction, for gravimetric methods).
Mass of dried HA: m(HA), g (the precipitate of the acid extraction, for gravimetric methods).The extraction yield of FA and HA is calculated by the next expressions:
Where:
Yield (FA): Extraction yield FA
Yield (HA): Extraction yield of HA.
V (FA): Volume of FA (mL)
ρ (FA): Density of FA (1.11 g / mL) (from a Gay Lussac 25 mL pycnometer)
m (HA): Mass of HA extracted (g)
m (RM): Mass of raw material (0.5 g)
2.4. Statistical analysis
In order to evaluate the differences between the means, for a probability value < 0.05, is used the multivariable ANOVA analyses and the Duncan multiple range test. The Pareto Diagram allows the determination of factors influence. To process the information is used the statistical package Statgraphics Plus Version 5.1.
2.5. Economic analysis
To determine if the proposed extraction alternatives are economically feasible, a preliminary calculation of the gross profit is carried out, to produce 1kg of HA (WHA). The gross profit (GP) is calculated from eq. (3), taken into acount the result of the sale of the products (HA and FA), PV, as well as the costs of the auxiliary materials consumed, MC (NaOH, KOH, HCl, H2SO4, H2O):
The flows of FA and HA produced and auxiliary materials consumed (basic extractant, acid extractant and water) in each alternative are calculated by eq. (4)
Where:
WX: flow of each stream (FA, basic extractant, acid extractant and water), kg/h
m(X): mass of each current consumed or produced, g
The amount of water consumed will be the sum of the water consumed in each extractant to achieve the desired concentration and that which must be added to the basic extraction to guarantee the solid/liquid ratio.
3. Results and discussion
3.1. Characterization of raw materials
The content of organic matter (OM) is of vital importance in these sources, due to the lack of this component in the soils, caused by the irrational use of chemical products [1], being the component of interest of humic acid.
Compos and vermicompost have higher organic matter content than garden waste, which makes them valuable sources of HA matter [5].
Table 3 Chemical characterization of raw materials.
| Parámetros | Raw material | ||
|---|---|---|---|
| VC | C | GW | |
| Humidity (%) | 35,19 | 41,37 | 59.81 |
| Organic matter (%) | 43,93 | 46,86 | 39.27 |
| Phosphorus (mg/L) | 0,87 | 0,95 | 0,92 |
| Potassium (mg/L) | 0,72 | 0,61 | 0,75 |
| Sodium (mg/L) | 0,38 | 0,36 | 0,42 |
| Calcium (mg/L) | 2,79 | 2,80 | 2,96 |
| Magnesium (mg/L) | 1,23 | 1,18 | 1,29 |
Source: Own elaboration
The garden waste has a higher humidity content, which causes a higher consumption of raw material and favors a lower consumption of water, depending on the solid-liquid ratio used in the basic extraction.
The composition of Na, Ca, Mg, P and K do not differ considerably between the different sources.
3.2. Basic extraction stage
At this stage the volume of HS extracted and the amount of separated humin are analyzed.
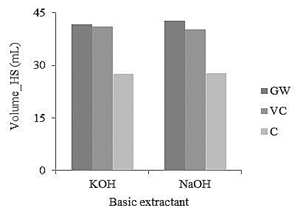
The highest amount of HS extracted (43 mL) is obtained from garden waste with NaOH as the basic extractant (see Table 4). Meanwhile, the lower volume extracted (4,87 g) is obtained from the compost, a product where the organic matter decomposes slowly, and that process depends on several factors, such as temperature, O2 supply conditions and pH [16].
Table 4 Responses variables for all alternatives.
| Alt. | V HS (mL) | m (H) (g) | V(AE) (mL) | V FA (mL) | m (HA) (g) |
|---|---|---|---|---|---|
| 1 | 43.00 | 4.84 | 0.50 | 41.00 | 1.41 |
| 2 | 42.00 | 4.93 | 0.10 | 40.00 | 1.35 |
| 5 | 40.00 | 4.80 | 0.20 | 38.00 | 1.42 |
| 6 | 27.00 | 5.16 | 0.20 | 43.00 | 1.51 |
| 3 | 42.00 | 12.65 | 0.10 | 40.00 | 1.23 |
| 9 | 43.00 | 12.21 | 0.30 | 38.00 | 1.51 |
| 7 | 39.00 | 13.31 | 0.30 | 40.00 | 1.64 |
| 8 | 40.00 | 12.02 | 0.40 | 39.00 | 1.93 |
| 4 | 41.00 | 27.21 | 0.30 | 23.00 | 1.23 |
| 10 | 28.00 | 22.40 | 0.20 | 19.00 | 1.22 |
| 11 | 28.00 | 22.03 | 0.80 | 21.00 | 1.70 |
| 12 | 27.00 | 23.15 | 0.40 | 23.00 | 1.80 |
Source: Own elaboration
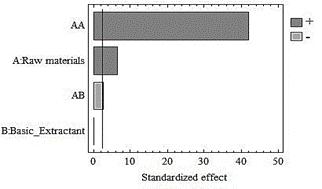
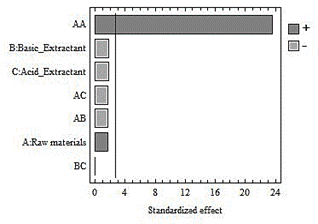
In Fig. 1, it is observed that the type of raw material influences inthe volume of HS extracted. The Duncan test shows that the fractions of HS from the garden waste differ from the other two (vermicompost and compost) (Table 5). This shows that this raw material content the highest proportion of decomposed organic matter [17].
Table 5 Variance analyzes for responses variables and Duncan test.
| Factor P-value | Responses variables | ||||
|---|---|---|---|---|---|
| V (HS) | m (H) | V(AE) | V(FA) | m (HA) | |
| RM | 0.004 | 0.000 | 0.460 | 0.0000 | 0.396 |
| VC | a | a | a | a | a |
| C | ab | b | a | b | a |
| GW | c | c | a | ac | a |
| BE | 1.000 | 0.490 | 0.020 | 0.160 | 0.941 |
| NaOH | a | a | a | a | a |
| KOH | a | a | b | a | a |
| AE | - | - | 0.340 | 0.160 | 0.967 |
| HCl | - | - | a | a | a |
| H2SO4 | - | - | a | a | a |
| The letters a, b and c define the homogeneity between the nivels of each factor. | |||||
Source: Own elaboration
The raw material-garden waste combination (GW-NaOH) matches with the highest HS volume extracted, therefore, it is where the greatest conversion of the basic reaction occurs (Fig. 2).
The type of raw material influences on the amount of humin removed, because the P-value is less than 0.05. The Duncan test shows that the results are different for the three raw materials (Table 4). The lowest amount of humin (4.60 g) is obtained from garden waste. It corresponds with the conditions in which the largest volume of HS is extracted, as shown in Fig. 2.
3.3. Acid extraction stage
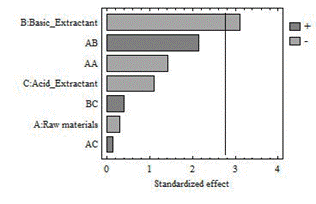
The volume of acid extractant to be added in the separation stage of humic and fulvic acids depends on the type of basic extractant used in the previous step, since in the analysis of variance the p-value of this factor is equal to 0.02 (Table 5). When sodium hydroxide is used, more acid extractant is consumed, based on the Law of the chemicals combinations [17].
The amount of FA extracted depends on the type of raw material. The Duncan test shows that the results for the compost are significantly different from those obtained from the vermicompost and the garden waste (Table 5). The Fig. 3 shows that the effect of interaction between the raw materials has a significant influence. The highest volume of FA (43 mL) is obtained in the alternative 6 (garden waste - NaOH - HCl), which was used by the International Humic Substances Society [13]. While the lowest volume is appreciated with the combination compost - NaOH - H2SO4; it matches with what is explained in the basic extraction stage (in epigraph 3.1).
The Fig. 4 shows that the type of basic extractant used influences on the amount of extracted HA. The highest amount of HA (1.93 g) is obtained in the alternative 8 (VC-NaOH-HCl) (see Table 3). These extractants have been used by the International Humic Substances Society because they are considered stronger [13].
The vermicompost is the raw material where the decomposition process of organic matter occurs faster, due to the microbial action exerted by earthworms. The earthworms fragment the organic waste substrates, stimulate microbial activity and increase the mineralization rates. This causes a quick conversion the wastes into humus-like substances with a finer structure than composts. [18].
3.4. Extraction yield of FA and HA
The maximum value of the percentage yield of AF extraction (9.65%) is obtained in alternative 6 (Table 6), with the material garden waste with sodium hydroxide and hydrochloric acid as basic extractants and acids respectively.
Table 6 Extraction yield of FA and HA for all alternatives.
| Alt. | Extraction yield (%) | Alt. | Extraction yield (%) | ||
|---|---|---|---|---|---|
| FA | AH | FA | AH | ||
| 1 | 9.09 | 28.21 | 7 | 8.81 | 32.73 |
| 2 | 8.91 | 26.92 | 8 | 8.58 | 38.53 |
| 3 | 8.88 | 24.53 | 9 | 8.51 | 30.13 |
| 4 | 5.11 | 33.93 | 10 | 4.14 | 24.33 |
| 5 | 8.44 | 28.41 | 11 | 4.59 | 34.07 |
| 6 | 9.65 | 30.28 | 12 | 5.03 | 35.93 |
Source: Own elaboration
The highest extraction yield value of HA is 38.53%, obtained in alternative 8 (VC-NaOH-HCl), higher than shown by [13] who reported 13.67%. The vermicompost is the raw material with the highest amount of organic matter (35-60%), agreed to data reported by the Provincial Laboratory of Soils in Matanzas (2020). [17] developed a preliminary experimentation of extraction of HA from VC, which can be considered an example of green technology, which will help to a sustainable, environmental-friendly and ecological agriculture.
3.5. Economic evaluation of alternatives
The Table 7 shows the reagents quantity and costs for alternative.
Table 7 Reagents quantity and costs for alternative to produce 1kg of HA.
| Alt. | BE | AE | H2O | TOTAL | |||
|---|---|---|---|---|---|---|---|
| W | MC | W | MC | W | MC | MC | |
| 1 | 1.42 | 0.11 | 0.35 | 0.24 | 19.67 | 0.03 | 0.38 |
| 2 | 3.38 | 2.54 | 0.06 | 0.00 | 5.38 | 0.01 | 2.55 |
| 3 | 3.71 | 2.78 | 0.15 | 0.27 | 8.93 | 0.01 | 3.07 |
| 4 | 2.68 | 2.01 | 0.07 | 0.00 | 5.92 | 0.01 | 2.03 |
| 5 | 3.20 | 2.40 | 0.11 | 0.22 | 6.65 | 0.01 | 2.63 |
| 6 | 1.32 | 0.10 | 0.12 | 0.01 | 9.91 | 0.02 | 0.12 |
| 7 | 1.22 | 0.09 | 0.17 | 0.20 | 9.73 | 0.02 | 0.31 |
| 8 | 1.04 | 0.08 | 0.12 | 0.01 | 10.43 | 0.02 | 0.10 |
| 9 | 3.02 | 2.26 | 0.12 | 0.01 | 10.01 | 0.02 | 2.29 |
| 10 | 3.74 | 2.80 | 0.15 | 0.13 | 8.56 | 0.01 | 2.94 |
| 11 | 1.17 | 0.09 | 0.43 | 0.10 | 23.67 | 0.04 | 0.22 |
| 12 | 1.11 | 0.08 | 0.13 | 0.01 | 11.19 | 0.02 | 0.11 |
Source: Own elaboration
Alternatives 2, 3, 4, 5, 9 and 10 have the highest material costs, because they use KOH as the basic extractant, which is more expensive than NaOH.
The alternative 5 (garden waste - KOH - H2SO4) is the most economic feasible, with 76.95 $/h of gross profit (see Table 8). For the compost case, the KOH-HCl ratio (alternative 4) is the one that reports higher values of gross profit, while for the vermicompost is the alternative 7 (NaOH - H2SO4). The most appreciable costs correspond to the acid extractants used (HCl and H2SO4).
Table 8 Products quantity and the gross profit for alternative to produce 1kg of HA.
| Alt. | FA | HA | GP | ||
|---|---|---|---|---|---|
| W | MC | W | MC | ($/h) | |
| 1 | 32.28 | 56.49 | 1.41 | 16.29 | 72.41 |
| 2 | 32.89 | 57.56 | 1.35 | 15.55 | 70.56 |
| 3 | 29.70 | 51.98 | 1.23 | 14.17 | 63.08 |
| 4 | 31.61 | 55.32 | 1.70 | 19.60 | 72.89 |
| 5 | 36.10 | 63.18 | 1.42 | 16.41 | 76.95 |
| 6 | 27.93 | 48.88 | 1.51 | 17.49 | 66.24 |
| 7 | 27.07 | 47.37 | 1.64 | 18.90 | 65.97 |
| 8 | 24.43 | 42.75 | 1.93 | 22.25 | 64.90 |
| 9 | 20.76 | 36.33 | 1.51 | 17.40 | 51.44 |
| 10 | 17.29 | 30.26 | 1.22 | 14.05 | 41.37 |
| 11 | 13.71 | 23.99 | 1.70 | 19.67 | 43.44 |
| 12 | 14.18 | 24.82 | 1.80 | 20.75 | 45.46 |
Source: Own elaboration
It is important to note that this economic analysis does not take into account production costs, so these results are not definitive for an industrial scale implementation.
4. Conclusions
In the stage of basic extraction of the humic substances, the type of raw material used was very important, the highest HS volume values were obtained with the garden waste. On the other hand, the type of basic extractant influences on the fractionation stage of the humic and fulvic acids (acid extraction). The alternative 8 (Tables 6 and 8) (Vermicompost - NaOH - HCl) is the most economic and technically feasible, with 38.53 % of extraction yield value of HA and 64.90 $/h of gross profit.