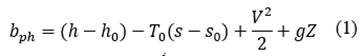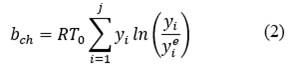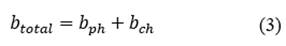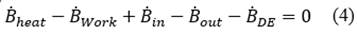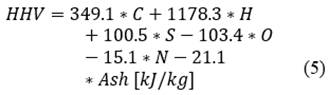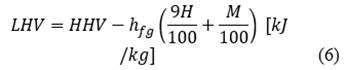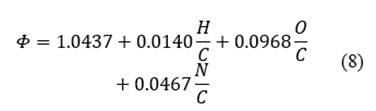1. Introduction
Due to the lack of effective economic-productive policies and strategic commitment to sustainable development, the agroindustry in Peru and in particular in Arequipa is still predominantly primary, with almost inexistent added value in its production processes [1]. Thus, the potential of agricultural products is wasted; including orange, a traditional crop with a national production of approximately 165,100 tons in 2016 [2]. In addition, the use of orange derivatives could contribute to the economic growth of the country in the form of new products.
Among the main by-products that can be obtained from orange are the pulp, pectin [3], essential oil [4] and flower water. However, there are no references to orange processing plants in the Arequipa area that make a comprehensive use of the fruit. But, in nearby areas there are lemon processing plants, where juice is produced from the pulp and essential oil of the peel.
As the orange is a typical crop of the region, which has high production rates, the use of the orange to provide added value to its by-products is a socio-economic opportunity that allows generating a contribution to the economic growth of the region. Thanks to this, a research project called Evaluación Integrada con Criterios de Sustentabilidad, del Proceso de Extracción por Arrastre de Vapor de Aceite Esencial de Cáscara de Naranja (Citrus Sinensis) de los Valles de la Provincia de Arequipa, en la Perspectiva de su Utilización Comercial was carried out in order to study the production of essential oil from the orange peel, with a view to the construction of a pilot plant for the production of orange essential oil with sustainability requirements in the use of material and energy resources.
Thus, the use of this fruit for the production of essential oil opens the possibility of processing other by-products. The pulp can be processed for the production of orange juice which is a common product in human consumption; the pectin found in the shell can be used for the production of biodegradable films for the manufacture of bags [5]; The essential oil, which is located in the peel (mainly D-Limonene), has components of multiple applications at the domestic and industrial level, with wide profitability margins [6]; and flower water, which is a by-product from the extraction of essential oil through steam entrainment, where the water, when it comes into contact with the essential oil, acquires certain odoriferous qualities that can be used for flavoring elements. Associating the above with the requirement of sustainability of the process, the need arises to evaluate a plant for the integral use of the orange outside the aforementioned project, from the exergetic point of view to guarantee the optimal use of energy resources.
Currently, works associated with the evaluation of processes from the exergetic point of view have been reported. As an example, in [7-9], They focus on the use of exergy to improve a process either by regenerating energy or finding areas of energy loss. In order to use exergy for optimization, minimizing the destroyed exergy, by proposing different scenarios, can be an opportunity to maximize the use of resources by reducing the irreversibilities. Thus, investigations of case studies are mainly focused on optimizing processes by using the destroyed exergy criterion. The specific use of destroyed exergy has been used as an optimization criterion in refrigeration, energy generation and coke production[10-12]. The criterion of minimum destroyed exergy has also been established as a selection methodology for a strategy as in[13]. The use of equation solvers like EES (Engineering Equation solver) to model a process involved in a destroyed exergy evaluation has been reported, in [14] an optimization of a combined cycle system based on destroyed exergy is presented. In the investigation process, the environmental and performance aspects of the system were considered, and exergy destruction is incorporated.
Taking into account the considerations above mentioned, the aim of this work is to evaluate a pilot plant for the integral use of oranges in terms of energy and exergy to process 120 kg/h of oranges, by generating different scenarios in order to minimize the destroyed exergy, from a base plant already established in the research project “Evaluación Integrada con Criterios de Sustentabilidad, del Proceso de Extracción por Arrastre de Vapor de Aceite Esencial de Cáscara de Naranja (Citrus Sinensis) de los Valles de la Provincia de Arequipa, en la Perspectiva de su Utilización Comercial”. These scenarios will be evaluated from the exergetic point of view, and from this, the desirable operating conditions will be determined using as a criterion the minimization of the destroyed exergy.
2. Material & methods
2.1. Description of the plant
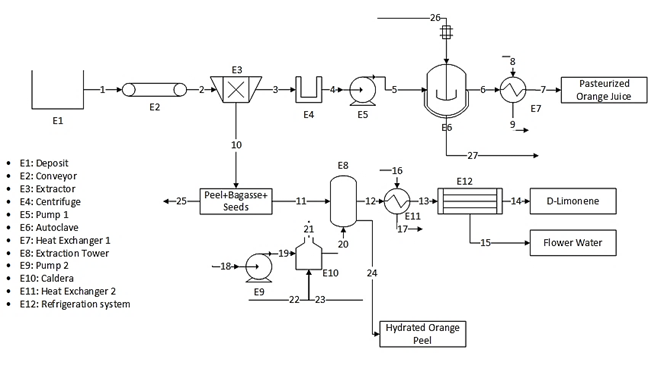
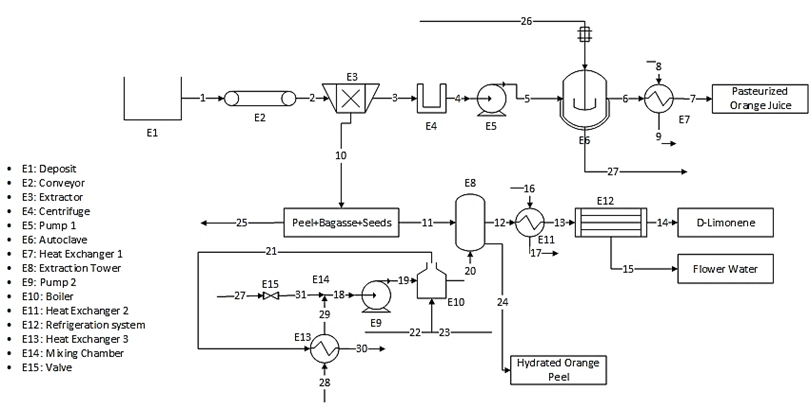
The plant consists of a reception area where the orange is delivered, deposited and pre-washed to remove the elements with which it comes from the crop (leaves, soil, etc.). After this, the fruit goes to a conveyor where it is classified and the one that is in poor condition is eliminated. At this point, the aim is to set an average flow rate of 120 kg/h after removing the fruit in poor condition. Once this is done, the fruit is ready for the extraction of the juice from the pulp, which is approximately 50% of the total mass of the fruit, while the peel produced is used later.
The purification of the juice requires removing the solid elements it contains. This is done, initially, in a clarifier which removes the larger particles and then the juice must be centrifuged to remove the smaller ones. At this point, the bagasse and seeds are recovered for later use [15]. The juice then goes to an evaporator where it is preheated and held at pasteurization temperature. Subsequently, the juice goes through the evaporation stages of the process where it is concentrated up to 66°Brix. The process is carried out in an autoclave to finally obtain a pasteurized orange juice ready for packaging [15].
On the other hand, the recovered peel, which represents approximately 10% of the total orange mass [1], is introduced into an extraction tower where a separation process by steam distillation is carried out. This step requires enter steam at a rate of 6.5 times the amount of orange peel per hour, that is, approximately a flow of 78 kg/h of steam to process 12 kg of orange peel of which approximately 3% by weight is essential oil [1].
Steam is generated by a pump that raises the water pressure to 400 kPa to mobilize it towards a boiler that will raise its temperature until reaching the state of saturated steam. The steam enters the tower to carry out the drag of oil. Up to this point, an emulsion of essential oil and steam has been obtained that needs to be cooled in order to separate it. In addition, there are losses in the amount of water due to the hydration of the orange peel of approximately 4% of the water used in the extraction.
The cooling process is carried out in two steps. First, the temperature of the emulsion is lowered through a heat exchanger so that it decreases until it reaches temperatures close to 35 °C, that is, 10 °C above ambient temperature. The second step consists of a refrigeration system that allows a higher separation performance, bringing the emulsion to a temperature close to 5 °C. At this point, two by-products are obtained: orange essential oil, which accounts for 0.5% of the total mass of the emulsion, and whose composition is mainly D-limonene; and flower water.
Finally, the wet orange peel that was used in oil extraction, which contains approximately 60% water and 5% pectin and negligible traces of oil, is planned to be used in a biofilm production process, which has not currently been designed, so the peel is considered a by-product to be exploited. Fig. 1 shows the process flow diagram of the integral orange extraction plant.
2.2. Plant modeling
All the modeling of the plant was carried out in EES, the considerations of the established plant model and the different scenarios will be presented:
The environment is at 25 °C and 80 kPa.
The feed water is at environment conditions
The demand for fuel is calculated from the need for heat required in the boiler.
The boiler works with 20% excess air and pure methane.
The equipment in the process is adiabatic and so do not present heat losses.
The properties of streams with water and other elements are evaluated separately, since there are no chemical interactions that modify the properties.
The specific heats of orange, orange peel and limonene are constant.
The Coefficient of Performance (COP) of the refrigeration system is considered 1.5, to establish the worst case for cooling.
The exergy of the organic elements other than the shell is 18.7 kJ/g for the detritus [16].
The efficiency of the pumps is 80%.
The essential oil in the orange peel is totally depleted.
The extraction power of 0.75 kW for a 120 kg/h process (Commercial Orange Juicer reference TT-J112A).
The temperature of the combustion gases leaving the first preheater is 200 ° C.
A throttle valve is used to reduce the pressure of the autoclave heating system.
In the second case, the outlet temperatures of the heat exchanger 4 are the same.
2.3. Exergy and reference environment
The reference environment is defined below in Table 1.
Table 1 Reference environment
| Property | Value | Units |
|---|---|---|
| Pressure ( P 0 ) | 101.3 | kPa |
| Temperature ( 𝑇 0 ) | 25 | °C |
| Molar fraction of O2 ( 𝑦 𝑂 2 ) | 0.2059 | - |
| Molar fraction of ( 𝑦 𝑁 2 ) | 0.7748 | - |
| Molar fraction of ( 𝑦 𝐶 𝑂 2 ) | 0.0003 | - |
| Molar fraction of H2O ( 𝑦 𝐻 2 𝑂 ) | 0.0190 | - |
Source: Adapted from [17].
The physical exergy as a function of the mass for each state is calculated from eq. ((1) presented by [17,18]. The chemical exergy of water and methane are tabulated in [19] with a value of 3120 and 836510 kJ/kmol respectively, and the chemical exergy of the combustion gases is calculated from eq. ((2) presented by [18,19], where 𝑇0 is the temperature in the reference state, 𝑦𝑖 is the mole fraction of the element in the mixture and 𝑦𝑖 𝑒 is the mole fraction of the element in its reference state. The total exergy as a function of mass is the sum of the physical exergy and the chemical exergy as shown in eq. ((3). The calculation of the destroyed exergy will be made from a balance of total exergy shown in eq. ((4) and exergy for steady-state control volumes of [18-20].
The calculation of the chemical exergy of the orange peel (eq. (7) is performed using the lower heating value (eq. (6) from the higher heating value (eq. (5) presented in [21], using the composition of the Table 2 presented in [22] by the standard relation of chemical exergy (Φ) presented in [23].
Table 2 Ultimate and proximate analysis of orange peel.
| Component | Percentage (%) |
|---|---|
| Carbon | 49.59 |
| Hydrogen | 6.95 |
| Oxygen | 39.7 |
| Nitrogen | 0.66 |
| Sulfur | 0.06 |
| Chlorine | 0.001 |
| Ash | 3.05 |
| Moisture | 2.73 |
Source: Adapted from [22].
The value of the standard ratio of chemical exergy is normally close to 1, however in [23] approximations are presented for solid compounds of C, H, O, N. In eq. ((8)), the expression used to calculate the relationship is presented. As a result of the above, the value of the standard ratio of the chemical exergy of the orange peel is 1.049 and the lower calorific value is 19734 kJ/kg. Therefore, the value of the chemical exergy of the orange is 20691 kJ/kg.
2.4. Thermoeconomic evaluation
The thermoeconomic evaluation was carried out through the method shown in [24] and using applicable restrictions shown in [25]. Eq. ((9)-(10)) show the system of equations that must be proposed by subsystem. It should be noted that one of the conditions established to close the system of equations is that all products have the same external link coefficient, since all of these have the same relevance as a final by-product.
3. Results and discussion
3.1. Plant
From the description presented in Description of the Plant, taking into account the percentages and mass quantity data, and the scheme showed in Fig. 1, the base scenario of the plant is modeled. The power of the equipment is calculated from energy balances and consulted in the average values of equipment, which are presented in Table 3.
Table 3 Equipment power based on an orange processing of 120 kg/h.
| Equipment | Name | Power | Units |
|---|---|---|---|
| E3 | Extractor | 0.75 | kW |
| E5 | Pump 1 | 0.006168 | kW |
| E9 | Pump 2 | 0.008746 | kW |
| E12 | Refrigeration system | 1.774 | kW |
Source: Own Elaboration.
By defining all the thermodynamic states and establishing the power of the equipment included in the process, the destroyed exergy of the main equipment can be calculated as shown in Table 4.
Table 4 Destroyed exergy by equipment
| Equipment | Name | Destroyed Exergy | Units |
|---|---|---|---|
| E3 | Extractor | 1.363 | kW |
| E5 | Pump 1 | 0.001533 | kW |
| E6 | Autoclave | 0.8357 | kW |
| E7 | Heat exchanger 1 | 0.1092 | kW |
| E8 | Extraction tower | 4.187 | kW |
| E9 | Pump 2 | 0.002173 | kW |
| E10 | Boiler | 13.4 | kW |
| E11 | Heat exchanger 2 | 1.460 | kW |
| E12 | Refrigeration system | 1.733 | kW |
| Total | 23.09 | kW |
Source: Own Elaboration.
The fuel requirement calculated from the balances and modeling conditions for the baseline scenario was 2.01 kg/h.
It should be noted that all the processes that are evaluated present a positive destroyed exergy, that means, they are feasible processes in nature, and this is due to the fact that the values of the mass balances come from studies carried out in the project [1]. Now, among the established processes, there is an equipment that show the highest destroyed exergy. The exergy destroyed in the boiler is 13.4 kW and this is due to the thermodynamic changes in the process. This is because through the boiler flows all the steam flow that is used in the main thermal processes such as the autoclave for pasteurization and the extraction tower to obtain essential oil.
and flower water, and being a thermal process with heat transfer, there is a high generation of entropy. Thus, indicating that the evaluation approach should be the boiler and the processes linked to it. Having established the above, a first option to minimize the destroyed exergy is to establish an economizer in order to preheat the water, thus minimizing the exergy destroyed and the fuel consumption of the boiler.
3.2. First scenario: pre-heater
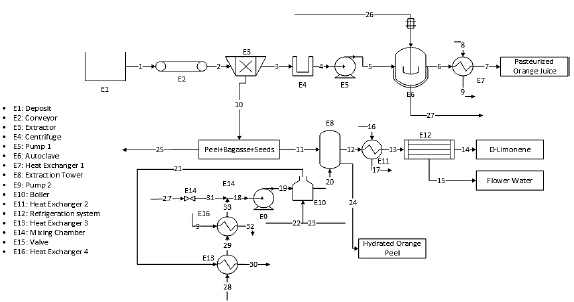
The first scenario consists of a pre-heater, whose function is to preheat the water consumed by the boiler using its combustion gases, on the other hand, the water used in the autoclave is redirected, which was used as a heat source for the pasteurization, and leaves said process at a temperature of around 60 ° C, to mix it with the preheated water and thus reuse the water used in the autoclave. The outlet temperature of the gases from this exchanger must be greater than or equal to 200 °C, recommended by The United States Environmental Protection Agency [26]. Fig. 2 shows the process flow diagram for the first proposed scenario.
As there is a change in the thermodynamic state of the boiler feed pump inlet (Pump 2), its power changes to 0.008048 kW in relation to presented in Table 3.
Thus, all the thermodynamic states have been defined and the power of the equipment included in the process established. The exergy destroyed of the main equipment can be calculated and this is presented in Table 5. A decrease in the exergy destroyed of 9.85% can be observed compared to the baseline scenario.
Table 5 Destroyed exergy by equipment for the first scenario
| Equipment | Name | Destroyed exergy | Units |
|---|---|---|---|
| E3 | Extractor | 1.36 | kW |
| E5 | Pump 1 | 0.00 | kW |
| E6 | Autoclave | 0.84 | kW |
| E7 | Heat exchanger 1 | 0.11 | kW |
| E8 | Extraction tower | 4.19 | kW |
| E9 | Pump 2 | 4.40E-04 | kW |
| E10 | Boiler | 12.08 | kW |
| E11 | Heat exchanger 2 | 1.46 | kW |
| E12 | Refrigeration system | 1.73 | kW |
| E13 | Heat exchanger 3 | 1.24 | kW |
| E14 | Mixing chamber | 7.82E-05 | kW |
| E15 | Valve | 6.28E-04 | kW |
| Total | 23.01 | kW |
Source: Own Elaboration.
Furthermore, one of the additional consequences of preheating is the decrease in the exergy destroyed and the power consumed in the boiler feed pump (E9). This is due to the fact that preheating occurs before entering the pump, due to the combination of the hot water outflow from the autoclave and the remaining flow demanded from the public network. This causes the destroyed exergy in this pump to decrease by 79.75%. A second consequence to highlight in this case is the decrease in fuel consumption due to preheating, which goes from 2.2 kg/h to 2.09 kg/h of methane.
In this way, the first case presented as a tentative scenario for the plant, it presents a significant response compared to a case of an economizer for the preheating of the boiler. However, this proposal opens an alternative to a second option. Under the established conditions, the temperature reached by the water is 54.44 °C, and the outlet temperature of the water from heat exchanger 1 (E7) is 85 °C. Taking this into account, there is a possibility to continue preheating the water using another pre-heater.
3.3. Second scenario: second pre-heater
In the second scenario, the water used to decrease the temperature at the exit of the pasteurization process, which reaches 85° C in the previous scenario, is used in a preheater to further increase the preheating temperature. Fig. 3 shows the process flow diagram for the proposed evaluation. Therefore, the idea is to increase, even more, the temperature at the entrance of the boiler.
Due to this change, and as expected, there is an even greater increase in temperature than that presented in the previous section for state 18 (from 54.9 to 66.91 ° C), and therefore a change in thermodynamic properties. Also, the boiler feed pump inlet (Pump 2) power changes to 0.007795 kW in relation to that presented in Table 3. Thus, all the thermodynamic states have been defined and the power of the equipment included in the process established. The exergy destroyed of the main equipment can be calculated and this is presented in Table 6.
Table 6 Destroyed exergy by equipment for the second scenario
| Equipment | Name | Destroyed exergy | Units |
|---|---|---|---|
| E3 | Extractor | 1.36 | kW |
| E5 | Pump 1 | 1.53E-03 | kW |
| E6 | Autoclave | 0.84 | kW |
| E7 | Heat exchanger 1 | 0.11 | kW |
| E8 | Extraction tower | 4.19 | kW |
| E9 | Pump 2 | 2.33E-04 | kW |
| E10 | Boiler | 11.68 | kW |
| E11 | Heat exchanger 2 | 1.46 | kW |
| E12 | Refrigeration system | 1.73 | kW |
| E13 | Heat exchanger 3 | 1.22 | kW |
| E14 | Mixing chamber | 8.15E-04 | kW |
| E15 | Valve | 6.28E-04 | |
| E16 | Heat exchanger 4 | 4.97E-02 | |
| Total | 22.64 |
Source: Own Elaboration
Taking into account the data presented in Table 6, a decrease in the exergy destroyed in the boiler can be observed compared to the base case and the first case study of 12.84 and 3.31% respectively, in other words, there was a significant decrease in the destroyed exergy of the boiler due to the configuration proposed. Regarding the exergy destroyed in the boiler feed pump (E9), there was a decrease in the destroyed exergy of 88.02% compared to the baseline scenario and 46.87% compared to the first scenario. Finally, regarding the fuel consumption of the boiler, this went from 2.2 kg/h for the base scenario to 2.04 kg/h for the current scenario. This makes this scenario best evaluated scenario since it minimizes the exergy destroyed, both of the plant and of the equipment that presents the greatest destruction, and likewise bringing benefits such as the decrease in fuel consumption.
In addition, an evaluation of the first a second law efficiencies were carried out in the main affected equipment, the boiler, the efficiencies are showed in Table 7. It is shown, that the second law efficiency increases as the destroyed exergy decreases. Also, the first law remains constant in all case, perhaps, because although exergy destruction is minimized, this is compensated by a decrease in mass flow.
3.4. Thermoeconomic analysis
As an addition to this research, it is interesting to develop a thermoeconomic analysis that allows establishing the economic effects that the different case studies have on the final products. However, as it is a methodology for development in an industrial process at the international level, the methodology of coefficient of external bonds is used. In this way, the increases in unit costs due to the exergetic changes in the system are established.
To meet the requirements of the thermoeconomic evaluation of the base plant, it is established that the coefficients of the inputs necessary for the development of the plant are equal to 1. As for the currents that are products that will not have use, the values corresponding to their coefficients are 0, and in this way load the weights of the exergetic components on the final products. Finally, to close the equations system the premise used is that all the by-products have the same coefficient, since they all have the same importance.
Table 8. shows the Coefficient of external bonds and exergy costs of each by-product. Now, as expected from the configuration with the least amount of exergy destroyed, it presents the least value of the products compared to the inputs. In this way, it can be shown that a decrease in the destroyed exergy causes a decrease in the exergy costs of the system. However, the decrease in exergy costs compared to the base case of close to 1.033%.
Table 8 Coefficient of external bonds and exergetic costs of by-products.
| By-product | Base scenario | First scenario | Second scenario | |||
|---|---|---|---|---|---|---|
| Coefficient of external bonds | Exergetic cost kW | Coefficient of external bonds | Exergetic cost kW | Coefficient of external bonds | Exergetic cost kW | |
| Pasteurize Orange Juice | 7.649 | 22.2 | 7.59 | 22.03 | 7.57 | 21.97 |
| D-Limonene | 7.649 | 14.31 | 7.59 | 14.19 | 7.57 | 14.16 |
| Flower water | 7.649 | 28.45 | 7.59 | 28.23 | 7.57 | 28.15 |
| Hydrated orange peel | 7.649 | 175.4 | 7.59 | 174 | 7.57 | 173.6 |
Source: Own Elaboration
4. Conclusions
In the present investigation, an evaluation of a pilot plant for the integral use of orange in terms of energy and exergy was carried out. This evaluation was based on two study scenarios proposed from a base plant for the integral use of the orange, with the aim of solving a need that arose in the project called “Integrated Evaluation with Sustainability Criteria, of the Process Extraction by Steam Drag of Essential Oil from Orange Peel (Citrus Sinensis) from the Valleys of the Province of Arequipa, in the Perspective of its Commercial Use”.
The first scenario evaluated was the use of an economizer in order to use the combustion gases from the boiler to preheat the water that enters it. However, the first scenario gave opportunity to the approach of the second study scenario, which was to use a second economizer that allows to continue increasing the inlet temperature of the water to the boiler.
In the second case, there was a greater decrease in the exergy destroyed both in the plant and in the equipment associated with the changes, and an even greater decrease in fuel consumption. Therefore, the optimal case is the second case study. As an addition to this research, a thermoeconomic analysis was developed to establish the exergy costs of the products for all the cases raised.
Regarding the thermoeconomic analysis, an evaluation was carried out for the three situations raised throughout the work presented, the evaluation was carried out using the methodology of the coefficients of external links presented by [24] and using the associated cost rates with the fuel and the product presented in [25] Thus, it is concluded that a decrease in the exergy destroyed generates a decrease in the exergy costs charged to the products, although this decrease was not a high percentage.