1. Introduction
The genus Pleurotus (Basidiomycota: Agaricals), also known as oyster mushroom, is a group of edible basidiomycetes that ranks third in mushroom production worldwide 1. The mushrooms are considered a functional food, which can provide health benefits beyond the traditional nutrients they contain 2.
Interest in mushrooms of the genus Pleurotus has increased in recent years because they are associated with multiple health benefits such as ability to modulate the immune system, hypoglycemic and antithrombotic activities; reduction of blood pressure and blood cholesterol. Moreover, these plant species also exhibit anti-inflammatory, antimicrobial, and anti-tumoral properties 3.
These mushrooms are appreciated for their taste and high nutritional value; they are a good source of dietary fibers and protein (20-35% d.b), although the lipid content is low (0.62-0.84% d.b). They also contain essential amino acids, minerals, and antioxidants 2. Edible mushrooms, Pleurotus sp., are also used to prepare soups, fries, and fillings. They can be sautéed, grilled, stewed, or boiled, and are important ingredients in vegetarian dishes 1. One of the limitations to the mushrooms use is that they are highly perishable due to their high moisture content and high post-harvest respiration rate. Thus, a drying procedure may be an option to extend their shelf life. Drying is a method traditionally used to process edible mushrooms, and the application of natural, solar, spray, hot air, vacuum, microwave, and/or freeze-drying has been studied 4,5. Since mushrooms are temperature-sensitive, it is necessary to choose drying conditions that minimize sensory and nutritional changes in the final product. Mushroom flour can be used in different food systems such as bakery products because they confer a high nutritional value and can be consumed by a wide variety of users 6. Morris-Quevedo et al., 7 cultivated edible mushroom (Pleurotus ostreatus) in coffee pulp; once harvested, the mushrooms were sliced, dried at 45°C, and milled. The flour was used to increase the nutritional and antioxidant properties of cookies by substituting 10% of the wheat flour.
The aim of this study was to characterize the edible mushroom Pleurotus sp., and to evaluate the physicochemical, antioxidant, and techno-functional properties of flours obtained by hot air drying at two temperatures: 50 and 60°C. The development and characterization of this material are important to fulfill the consumption trends on foods derived from vegetable protein and to obtain a novel food ingredient for functional foods, which is barely used in bakery products. Moreover, the drying technology leads to increase drying velocity and consequently to obtain better quality properties and consume less energy.
2. Materials and methods
2.1. Raw material
Edible mushrooms of the genus Pleurotus sp. were supplied by the company Umay Parque Agrícola (Santa Elena, Antioquia). Pleurotus sp was grown in a substrate composed by a mixture of agro-industrial residues (bean pods, wheat bran, corn straw, coffee husk, wood sawdust, wood chips, and rice husks) and lime. They were disinfected with a solution of ACID-TEC (Lidetec, Medellín, Colombia) prior to characterization.
2.2. Physicochemical characterization of the mushroom Pleurotus sp
The moisture content was determined using a moisture measurement scale (Kern, model DAB 200-2, Germany). The mushroom was cut horizontally and vertically simulating a grid. Approximately 1 g of sample was weighed and analyzed on the scale at 105°C. A dew point hygrometer (AquaLab TE series 3B v3.52, Bogotá, Colombia) and the AOAC 978.18 methodology were used to determine water activity (aw) 8. The mushrooms were cut horizontally and vertically simulating a grid, then placed in cells, and the aw was measured.
The pH measurement was performed according to AOAC 943.02 9. Thirty grams of fresh mushroom were homogenized in 90 mL of distilled water for 2 min using a blender (Oster BLST4655 Classic silver, Bogotá, Colombia) at maximum speed (3). The sample was filtered using Whatman Paper No. 1. Subsequently, 10 mL of the filtrate were employed to measure pH at room temperature using a potentiometer (Hanna Instruments model 211, Bogotá, Colombia). Titratable acidity (TA) (%) was determined according to the methodology described by Padhi et al. 10 with some modifications. Ten milliliters of the initial filtrate were used and 3 drops of 10% phenolphthalein in ethanol were added and titrated with 0.98 M NaOH solution under constant stirring to pH=8.1 (equilibrium or neutral point). The results were expressed in terms of citric acid. An aliquot of the initial filtrate was dropped onto the digital refractometer glass to determine °Brix (Hanna, HI 96801, Bogotá, Colombia).
The color of the fresh mushroom was determined using the methodology described by Cortés et al. 11. The measurement was carried out on the smooth (top surface of the cap) and gills (lamellae at the bottom of the cap) faces, using a colorimeter (Konika Minolta, CR-400/410, Mexico) illuminating D65 and 10° observer as reference. The color coordinates of the CIE-L*a*b were obtained from the reflection spectra, where L* is an indicator of luminosity, a* is green (-) to red (+) chromaticity, and b* is blue (-) to yellow (+) chromaticity. The saturation or chromium (C*) was calculated with these parameters.
The mushroom firmness was determined according to the methodology described by Cortés et al. 11. On the smooth face, a puncture test was performed using a texture analyzer (Stable Micro System, TA-XT2i, Madrid, España), 2 mm plunger tip, and a penetration rate of 4 mm/s up to 5 mm. A sample of 3 units of mushroom was taken and 3 readings were made at the top of the cap.
The crude protein content was determined by the volumetric method (Kjeldahl) according to the AOAC methodology (2.026) 12, the ash percentage by ISO 5984:2002, (13) and the fat content following the AOAC methodology 920.39 14.
2.3. Production of the mushroom Pleurotus sp. flour
The raw material was cut with a thickness of 6 mm to achieve an uniform drying. Mushrooms were dried in a hot air dryer with trays (ESA 7.5 series 1591, Mallas y Silos de Antioquia, Sabaneta, Colombia), which was connected to a propane gas source for heat generation coupled to a flue gas exchanger. Two drying conditions were used: at 50°C for 150 min, and 60°C for 105 min until obtaining a sample with a moisture content less than 10%. The drying curves were determined by weighing the samples in triplicate every 10 min until reaching a constant weight (system in hygroscopic equilibrium). Samples were ground in a coffee mill (Hamilton Beach, 80350RV, Wisconsin, EEUU) until obtaining a flour. This methodology was standardized by the researchers. The particle size was measured using a Ro-TAP equipment and following the AOAC methodology AOAC 965.22 15. The flour had a particle size of 159 µm and 150 µm for the mushroom dried at 50ºC and 60ºC respectively.
2.4. Physicochemical and technofunctional characterization of the mushroom Pleurotus sp. flour
Mushroom flour (5 g) was weighed, homogenized in 50 mL of distilled water, and stirred constantly for 30 min to determine pH and acidity. The samples were left at rest, and the supernatant was taken to conduct the measurements. The pH was measured according to AOAC 943.02 9 using a potentiometer (Hanna Instruments, 211, Bogotá, Colombia). The acidity was measured using the methodology described by Padhi et al. 10 with some modifications, i.e., 5 mL of a 1:5 supernatant solution, distilled water was titrated with NaOH (0.98 M) to pH ≈ 8.1. The acidity content was expressed as a percentage of citric acid.
The moisture content of the mushroom flour was determined by the official method AOAC 925.10 16, and the water activity (aw) was measured according to AOAC 978.18 8. The crude protein content, ash percentage, and fat content of the mushroom flour were determined by the methodologies described above.
The color of the mushroom flour was determined with a colorimeter (Konika Minolta, CR-400/410, Mexico) using a cell for flour material, illuminant D65 and 2° observer. L*, a *, b* values were measured. The values of whiteness index (WI), hue angle (°h), and chromaticity (C) were calculated according to the methodology proposed by Padhi et al. 10. The total color difference ΔE 17,18 was also calculated.
The bulk density (BD) was determined according to the methodology proposed by Salazar et al. 19 with some modifications; specifically, 15 mL centrifuge tubes were used. Carr indices and Hausner ratio were calculated according to the methodology proposed by Moravkar et al. 20.
Techno-functional properties such as water retention capacity (WRC) (g/g), swelling capacity (SC) (mL/g), and water solubility index (WSI) (%) were measured according to the methodology proposed by Heo et al. 21. Fat absorption (FA) (mL/g), emulsifying activity (EA) (%), and emulsion stability (ES) (%) were determined according to the methodology described by Wang and Kinsella 22 with some modifications. For EA and ES, 1.4 g of mushroom flour were weighed and added to 20 mL of distilled water, the solution was homogenized with an Ultraturrax (IKA, model T25, Argentina) at 5000 rpm for 1 min. Subsequently, 5 mL of sunflower oil were added and homogenized at 7000 rpm for 3 min. That emulsion was transferred to 15 mL centrifuge tubes in 2 equal parts. One of the samples was centrifuged at 1134 g at 25ºC for 5 min. The EA was determined as a percentage: the ratio of the height of the emulsion and the total height. Regarding the emulsion stability, the other aliquot was measured and taken to a water bath at 80°C for 30 min; then, it was cooled down to 15°C for 20 min; finally, the sample was centrifuged at 1134 g at 25ºC for 5 min. The ES was calculated as the percentage of the height of the emulsion over the total height. Foaming capacity (FC) (%) and foam stability (FS) (%) were evaluated following the methodology described by Yust et al. 23. 50 mL of 3% w/v dispersion of the sample were prepared in distilled water and homogenized using an (IKA, model T25, Argentina) at 7000 rpm for 3 min.
The total phenolic compounds were determined using the Folin-Ciocalteu reagent 24 with some modifications. Mushroom flour (1 g) was weighed into a 15 mL test tube, 8 mL of methanol:water solvent (70:30) was added, capped, and vortexed for 30 s at a rate of 3000 rpm. Subsequently, the sample was centrifuged (Universal 320R centrifuge, Tuttlingen, Germany) for 15 min at 7084 g and 25°C. The supernatant was filtered with 90 mm filter paper over a 25 mL volumetric balloon. The process with the precipitated sample was repeated twice. The volume of the obtained supernatants was adjusted to 25 mL with distilled water. The sample was taken into a 50 mL test tube previously covered with aluminum foil and stored at 4°C. To measure the total phenols, 100 µL of sample filtered with 400 µL of Na2CO3 were taken in a 2 mL centrifuge tube and vortexed at 3000 rpm for 10 s. Subsequently, 5 µL of the Folin solution were added and left in a dark place for 2 h until analysis. The absorbance of the samples was determined at 760 nm on a spectrophotometer (Thermo Fisher Scientific Inc, Evolution 60, Waltham, MA, USA). The extractable phenols content was expressed as mg gallic acid/g dry matter.
The Trolox as a standard and controlled temperature conditions at 37°C were used to determine ORAC (Oxygen Radical Absorbance Capacity). The methodology described by Ou et al. 25 was used for this test. The readings were made at an excitation (of 493 nm, and ( emission of 515 nm. Fluorescence was measured on a fluorescence spectrophotometer (PerkinElmer, Model: LS55, Waltham, MA, USA) and the radical scavenging of the antioxidant was calculated using the differences of areas under the fluorescein decay curve between the blank and the sample and compared to the Trolox curve. The results were expressed as TEAC values (µmol of Trolox/ g dry sample).
3. Results and discussion
3.1. Physicochemical characterization of fresh mushroom Pleurotus sp
The fresh edible mushrooms presented a protein, ash, and fat content of 2.5 g/100 g, 0.73 g/100 g, and 0.50 g/100 g, respectively. Jaramillo et al. 5 reported values of 0.61% (ash), 2.72% (protein) and 0.17% (fat) for the edible mushroom Pleurotus ostreatus. The mushroom used in this study had a lower amount of protein, but a higher content of ash and fat.
Carvalho et al. 1 reported contents of protein and fats in dry base (d.b.) between 11-42% and 0.2-8% for different species of Pleurotus. Picornell-Buendía et al. 26 obtained edible mushroom Pleurotus ostreatus with protein content between 17.02 to 19.49 g/100 g d.b., and ashes between 5.81 to 7.21 g/100 g according to the substrate in which they were grown. The differences in composition can be attributed to the different types of substrates where the mushrooms were grown. For instance, Dundar et al.27 grown Pleurotus ostreatus in substrates with different Carbon/ Nitrogen (C/N) relation such as wheat stalk (C/N 84.32), cotton stalk (C/N 150.70), millet stalk (C/N 77.38), and soybean stalk (C/N 72.14). That study reported that the mushrooms grown in soybean stalk had the highest protein content, and those grown in millet stalk had the lowest. The highest fiber and lipid content were found in millet stalk and the lowest in soybean stalk. Other types of substrates such as palm oil waste (shaft and bunch) and sawdust supplemented with wheat and rice bran were used in the study conducted by Elkanah et al. 28. The results indicated that the mushrooms grown in the shaft supplemented with wheat bran had the highest protein content. The highest lipids and fiber content corresponded to sawdust without supplements. Regarding the minerals content, the highest iron was found in bunch and sawdust; the highest potassium in the bunch, the highest sodium in the shaft, and the highest magnesium in the shaft and rice bran.
Moreover, it is important to note that the amount of nutrients may shift with respect to the evaluated part of the mushroom. Zhang et al. 29 reported that the nutrient content in the pileus (cap) was higher than that of the stipe (stem) of the edible mushroom Lentinus edodes.
Jaramillo et al. 5 reported moisture values similar to those found in this work, (92.64 g/100 g) in oyster mushrooms (Pleurotus ostreatus), while Yang et al. 30 found lower moisture values, between 86.73 ± 0.28 and 88.60 ± 0.65, in Pleurotus. The high moisture content makes them a perishable product, which is why conservation techniques such as drying are sought 5.
Cortes et al. 11 performed the physicochemical characterization of edible mushrooms of the variety Pleurotus ostreatus and reported values of aw (0.994 ± 0.002 to 0.995 ± 0.001), moisture (90.3 ± 0.9 to 94.3 ± 0.5%), pH (6.25 ± 0.05 to 6.47 ± 0.03), Brix (2.3 ± 0.3 to 4.3 ± 0.1), and acidity (0.15 ± 0.01 to 0.24 ± 0.02 g citric acid/100 g). Values close to those found in this research.
Picornell-Buendía et al. 26 reported firmness values between 219.08 and 281.68 N for P. ostreatus grown on different substrates; values higher than those found in this study. The differences among the firmness of the mushrooms may be related in part to dissimilarities in the used measurement methodologies, and/or to variations in the moisture content of the harvested fruiting bodies, as well as their harvest time.
The L* value for the surface of the cap was slightly higher than the rough side; however, both are in the range of a clear luminosity (70-79) 11. The color of the mushrooms was between cream to white, lighter on the surface of the cap and with a cream color on the rough side. The parameters L* and b* are lower on the rough side, whereas a* has higher values. For C* -i.e., color saturation, which is proportional to its intensity- Nölle et al. 31 found values of 18.91 ± 1.76 in Pleurotus ostreatus, higher than that on Table 1.
Table 1 Physicochemical characterization of fresh mushroom Pleurotus sp.
| Parameter | Value |
|---|---|
| Moisture (%) | 91.52 ± 0.71 |
| aw | 0.9942 ± 0.0067 |
| pH | 6.06 ± 0.26 |
| Acidity (% citric acid) | 0.228 ± 0.011 |
| °Brix | 3.45 ± 0.34 |
| Firmness (N) | 1.216 ± 0.246 |
| Cap's Smooth Surface Color | |
| L* | 76.02 ± 4.57 |
| a* | 0.51 ± 0.20 |
| b* | 13.30 ± 1.41 |
| C* | 13.31 ± 1.42 |
| Cap's Rough Surface Color | |
| L* | 71.34 ± 3.87 |
| a* | 1.05 ± 0.42 |
| b* | 10.53 ± 1.14 |
| C* | 10.59 ± 1.17 |
Source: Created by the authors.
Picornel-Buendía et al. 26 reported L* values between 71.24-73.99, a* between 2.16-3.09, and b* between 11.04-14.43 for P. ostreatus. The values of L* and b* were similar to those found in this research, while the parameter a* was superior. Variations in color parameters are attributed to factors such as the substrate used for planting, harvest conditions, and the composition of the substrate. The study made by Picornell-Buendía et al. 26 used a mixture of wheat straw (3,000 g) and Pleurotus substrate (3,000 g) supplemented with 120 g of each commercial supplement (Promycel® and Calprozime® Champfood®) rich in protein and mineral content which promoted red-green (a*) and yellow-blue (b*) chromaticity in harvested mushrooms.
3.2. Drying of the mushroom Pleurotus sp
Fig. 1A shows two phases. In the first one, a large amount of water is evaporated from the mushroom -about 50% of the initial weight of the product, mainly free water. In this period, the mass transfer is controlled by the drying operating conditions. This stage starts from time 0 until the end of the 20 min drying at the two temperatures. In the second phase, the mass transfer is controlled by internal resistances of the mushroom to reach equilibrium moisture. This stage begins at 40 min drying for the two process temperatures and ends at 90 min for treatment at 50°C and 80 min for drying at 60°C. Shanket et al. 32 reported a drying time of 90 min at a temperature of 50ºC and 70 min at 60ºC for drying Pleurotus ostreatus.
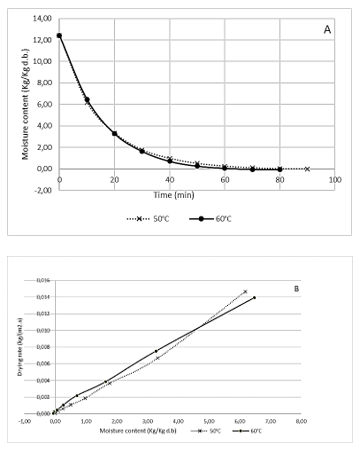
Source: Created by the authors.
Figure 1 Drying curve (A) and drying rate curve (B) obtained from Pleurotus sp. mushrooms during dehydration at two temperatures.
Fig. 1B shows zones with different drying rates for the two temperatures. Each zone has a decrease in drying rate with a decrease in moisture content. An effect of temperature on drying rate is also observed. Furthermore, the moisture content influences the drying rate; a high moisture content resulted in a high drying rate. In the final drying stage, where the moisture content is low, the two temperatures show different paths for similar moisture contents. As the drying process progresses, the temperature and moisture gradients between the mushroom slices and the surrounding hot air gradually decrease. Thus, the drying rate of the slices decreased as the moisture content was reduced after the initial heating. It was also possible to see an increase in the drying rate with the increase in the drying temperature, which implied an 11% reduction in the process time when the drying temperature went from 50 to 60°C. Apati et al. 33 found an increase in drying rate with increasing temperature, thus reducing the process time by 28.6% when applying a drying temperature of 60°C -instead of 50°C- to dehydrate P. ostreatus in an oven with hot air circulation.
Siti-Nuramira et al. 34, stated that cabinet drying is the optimal choice as a low-cost postharvest treatment because it reduced microstructure degradation of the mushrooms, retained more nutritional components, and increased shelf-life and yield.
Recent trends in mushroom drying technologies such as vacuum microwaves have been studied to increment drying velocity and consequently reduce energy consumption when combined with hot air drying 35.
3.3. Characterization of the mushroom Pleurotus sp flour
The mushroom Pleurotus sp. flour presented a protein content of 22 g/100 g, ash 9.48 g/100 g, and fat of 1.26 g/100 g. The relatively low-fat content could make it a potential raw material to formulate a variety of dietary food products. Also, its good protein content could be an economic source of protein for people in developing countries 36.
Salehi 6 reported a fat content of 2.3%, protein of 22.41%, carbohydrate of 60.47%, and ash of 7.79% with a moisture content of 7.04% for Pleurotus sajor-caju flours. These results show similar values for protein, a higher fat content, and lower ash than those found in this work. Moreover, Oluwaseun et al. 36 reported a protein content of 20.4 ± 0.3%, fat of 1.6 ± 0.1%, and ashes of 5.7 ± 0.1% for Pleurotus ostreatus flour.
Protein, fat, and ash contents of 27.4 ± 0.18, 4.04 ± 0.39 and 7.57 ± 0.49 g/100 g, respectively, have been reported for Pleurotus ostreatus flour 7; the first two values are higher than those found in this study. This is possibly related to the species of mushrooms and its growing conditions, especially the substrate. The higher ash content implies a relatively higher mineral content. Thus, it depends on the type of material, method of incineration, time and temperature used in the drying process, as well as the species and growing conditions 37.
Jaramillo et al. 5 found that Pleurotus ostreatus flour obtained by hot air drying at temperatures of 50 and 60°C had a protein content of 27.85 and 28.94%, respectively. These values are higher than those found in this work at the same drying temperatures. It should be noted that denaturation and variation in the content of most proteins is not likely at temperatures below 60°C.
Other drying technologies such as freeze drying and microwave have been used to obtain mushroom flour. The study of Priyadarsini et al. 38, compared hot air drying, freeze drying, and microwave; it reported more protein content in hot air drying at 40ºC (30.81%) than in freeze drying (29.53%).
No significant statistical differences were found for the evaluated physicochemical variables of mushroom flours (Table 2) except for moisture content, which was higher in dried mushrooms at 50°C. In addition, drying at 60°C generates higher temperature gradients between the mushrooms and the hot air and increases the diffusion of water from the center to the surface. Therefore, it yields a lower final moisture with respect to drying at 50°C. Moreover, the moisture content of the flour at 50°C is below 10% and together with the water activity, which is low (aw <0.6), they inhibit microbial growth so the product can be considered stable in storage under suitable packaging conditions. According to Oluwaseun et al. 36, flour-based foodstuffs with less than 13% moisture content are more stable to self-oxidation and deterioration, especially during storage. These authors obtained Pleurotus ostreatus flour by hot air drying at 60°C and a moisture of 7.5± 0.1, similar to that obtained in this study at the same temperature.
Table 2 Physicochemical characterization of the Pleurotus sp. flour obtained by drying at two temperatures.
| Parameter | H-50°C | H-60°C |
|---|---|---|
| Moisture (%) | 9.72 ± 0.98a | 7.62 ± 0.95b |
| aw | 0.382 ± 0.076a | 0.265 ± 0.056a |
| pH | 6.04 ± 0.07a | 6.09 ± 0.04a |
| AT (% citric acid) | 0.59 ± 0.00a | 0.58 ± 0.01a |
| L* | 46.26 ± 3.38a | 47.92 ± 3.08a |
| a* | 1.29 ± 0.51a | 0.47 ± 0.55a |
| b* | 18.02 ± 1.54a | 14.81 ± 1.83a |
| C | 18.07 ± 1.50a | 14.82 ± 1.84a |
| ΔE | 27.799 | 25.600 |
Different letters in the same row indicate significant differences (p<0.05). ΔE was calculated with respect to the average value of fresh mushrooms. TA: Titratable Acidity.
Source: Created by the authors.
Djamila et al. 37 obtained oyster mushroom flour by rotary vacuum drying at 60°C and sun drying with aw of 0.390 and 0.350, values close to those mentioned in Table 2. The lower the aw value, the longer the shelf life; whereas the higher the aw, the lower the storage capacity.
There were no significant statistical differences between the color of the flours obtained at different drying temperatures. Regarding the color parameters of fresh mushrooms, the luminosity decreases, probably due to browning effects during the drying process. Low L* values may affect the overall acceptability of mushroom flour 39, therefore, its use in other food matrices. Djamila et al. 37 reported an L* value of 60.71-63.05 for Pleurotus ostreatus flour obtained by sun drying (32°C) and vacuum drying (60°C), values higher than those of Table 2.
Fig. 2 shows the difference in color between the flours obtained at both temperatures with respect to the fresh mushroom. The ΔE has close values at both temperatures, a difference greater than 3, thus appreciable for the human eye 18. It indicates that the drying process affected the color. These changes may be due to both enzymatic and non-enzymatic browning reactions. The first ones are given by the presence and activity of the enzyme polyphenol oxidase (PPO), which can oxidize phenols and cause tissue darkening 40. The latter, Maillard reaction, may be given using higher drying air temperatures. The main cause of dried P. Eryngii darkening could be heat causing non-enzymatic darkening reactions that depend on the temperature and heating time during the drying process 17. Those researchers reported ΔE values of 23.7 ± 2.3 for mushrooms dried by hot air at 60°C, and L*, a*, and b* values of 54.5 ± 4.2, 1.4 ± 0.3, and 13.4 ± 2.4, respectively.

Source: Created by the authors.
Figure 2 Fresh Pleurotus sp. mushroom (a), fresh mushroom slices (b), mushroom dried at 50°C (c), mushroom dried at 60°C (d), mushroom flour dried at 50°C (e), and mushroom flour dried at 60°C (f).
Although no statistical differences were found among the color parameters, higher color preservation values were observed in the flour obtained at 60°C. Shanker et al. 32 reported similar results and found better color retention of oyster mushrooms dried by hot air at a temperature of 60°C, compared to 50 and 70°C. The greatest color loss at 50°C is attributed to the long drying time.
The temperature used for drying the edible mushrooms did not result in significant statistical differences for bulk density (Table 3). It depends more on the grinding process, which was the same for mushrooms dried at both temperatures. Zhang et al. 29 reported bulk densities between 0.150 ± 0.004 and 0.228 ± 0.009 g/mL for L. Edodes flour obtained from the cap and stem by different particle size reduction mechanisms. The authors found that the density was higher for flours obtained by shearing than by mechanical milling and jet milling. This is because the lower particle size has a greater surface and homogeneous contact with the environment, which decreases the spaces between particles and increases the density value. Ishara et al. 41 found values close to those of Table 3 for the bulk density of Pleurotus ostreatus and Agaricus bisporus flours, i.e., 0.28 ± 0.01 and 0.22 ± 0.00 g/mL, respectively. Meanwhile, Aremu et al. 42 reported density values for flours of three different edible mushrooms between 0.23 and 0.41 g/mL obtained by oven drying at 45°C for 36 h and sieved using a 1mm sieve.
Table 3 Bulk density, Carr index, and Hausner ratio and techno-functional properties of mushroom flour obtained at two temperatures.
| Property | H-50°C | H-60°C |
|---|---|---|
| Carr Index | 28.89 ± 3.86a | 33.00 ± 1.12a |
| Hausner ratio | 1.41 ± 0.08a | 1.49 ± 0.03a |
| BD (g/mL) | 0.33 ± 0.05a | 0.31 ± 0.03a |
| WRC (g/g) | 0.14 ± 0.02a | 0.14 ± 0.00a |
| SC (mL/g) | 5.762 ± 0.66b | 6.88 ± 0.25a |
| FA (mL/g) | 2.17 ± 0.26a | 2.48 ± 0.23a |
| WSI (%) | 33.52 ± 2.56a | 33.49 ± 1.87a |
| FC (%) | 11.51 ± 0.97a | 10.71 ± 1.89a |
| FS (%) | 5.82 ± 1.83a | 4.17 ± 0.76a |
| EA (%) | 26.49 ± 1.21a | 23.89 ± 6.99a |
| ES (%) | 26.84 ± 1.10a | 28.36 ± 0.70a |
Different letters in the same row indicate significant differences (p<0.05). Bulk density (BD), water retention capacity (WRC), swelling capacity (SC), fat absorption (FA), Water solubility index (WSI), foaming capacity (FC), foam stability (FS), emulsifying activity (EA) and emulsion stability (ES).
Source: Created by the authors.
The Carr index and the Hausner index of the mushroom flour dried at 50 and 60°C presented a qualification parameter for the fluidity variable between "poor" and "very poor"(Table 3). Therefore, the flour particles showed high cohesiveness and compressibility. If a material is highly compressible, its bulk density could increase and complications in movement may occur; this would probably end up in agglomeration 43.
Regarding the technofunctional properties, significant differences were observed only for the swelling capacity (SC), between the drying temperatures of 50 and 60°C. It was higher for Pleurotus flour at 60°C. Zhang et al. 29 recorded a SC similar to that reported in this study at 50°C for Shiitake's cap flour made by 3 grinding processes, values between 5.239 ± 0.058 and 5.769 ± 0.080 mL/g. They also report a WSI between 23.143 ± 1.01 and 27.358 ± 1.21%, which is below than that reported here. It could be related to the differences in the protein and fiber content of each mushroom flour.
Ishara et al. 41 reported a WRC of 6.27 ± 0.36 g/g for Pleurotus ostreatus flour, a value higher than that found in this research. They also reported a WSI (50.99 ± 6.67%) and SC (13.71 ± 0.28 mL/g), both values lower than those reported in this study for the two evaluated temperatures. This could be explained by the hydrophilic components of the material such as polysaccharides and proteins, which are related to diffusion and affinity for water. These properties are probably supported by the presence of fibers and by the porous morphology of the flours 41. Salehi 6 found WRC values of 13.46 ± 0.28 g/g for Pleurotus sajor-caju flour and SC 19.49 ± 0.24 mL/g, results higher than those presented in Table 3. Also, Oluwaseun et al. 36 found a SC of 2.9 g/mL for Pleurotus ostreatus flour, a value lower than that of this study. Low values may be related to the content of non-soluble dietary fiber and a lower amount of starch in the flour.
Food foams depend on surface activity and film-forming properties of specific protein components that stabilize them by unfolding at interfaces and then associate to form cohesive films around air cells 23. The foaming capacity (FC) and foam stability (FS) parameters help characterize foaming properties of protein solutions and are related to the whipping capacity. High values in these properties could indicate that the flour is suitable for food products that require a high percentage of porosity, such as cakes or frostings 41,42. In their research, Aremu et al. 42 reported FC values between 101.8 ± 4.0% and 131.48 ± 5.0% for three varieties of edible mushrooms Ganoderma spp., Omphalotus olearius, and Hebeloma mesophaeum, with a stability (FS) after 24 h between 51.0 ± 0.5% and 54.0 ± 1.0%, values higher than those found in the Pleurotus sp. flour evaluated here. Ishara et al. 41 found a FC of 18.86% for Pleurotus ostreatus, closer to that reported in Table 3.
According to them, fat absorption capacity is given by proteins in flour that can physically bind to fat by capillary attraction. This property plays an important role since fat acts as a flavor retainer and increases mouthfeel and palatability 42. They found a fat absorption capacity of 462.6 ± 109% for Pleurotus ostreatus flour, higher than that of this research -2.17 to 2.48 mL/g (199.21 to 227.66%)- and close to values reported for corn flour 42. Pleurotus sp. flour shows greater oil retention than water (Table 3), a fact that could indicate compounds with more lipophilic characteristics. According to Han et al. 39, the oil retention capacity can be attributed to the insoluble fiber content in the flour.
Emulsifying activity (EA) refers to the maximum amount of oil emulsified by the protein in the flour, or the ability of a molecule to act as an agent that aids in the solubilization or dispersion of two immiscible liquids 39. This property is useful to prepare baked goods, mayonnaise, and ground meat sausages 41. The emulsion stability (ES) is the ability to maintain the integrity of the emulsion 39. In the literature, values of approximately 49% for the EA of Pleurotus ostreatus flour and 32% for Agaricus bisporus flour, and an ES of 35% and 19%, respectively, were reported (41); values higher than those of this study except for the last one. For Pleurotus sajor-caju flour, Hanet al. 39 reported an EA of 51.67 ± 0.25% and an ES of 95.37 ± 0.21%, values higher than those reported in Table 3.
The total phenolic compounds in the mushroom flour did not show significant differences between drying temperatures. Total phenolic compounds are the most naturally abundant antioxidants in mushrooms including compounds like ferulic acid, p-hydroxybenzoic acid, p-coumaric acid, gallic acid, and cinnamic acid 44. Sahnker et al. 32 reported a phenol content of 2.67 ± 0.010, 2.77 ± 0.017 and 2.53 ± 0.010 mg GAE/ g dry base for Pleurotus ostreatus dehydrated at 50, 60 and 70°C, respectively; values slightly lower than those of this study. These authors indicate that the phenols contained in oyster mushrooms are highly sensitive to time, temperature, and drying method. According to Gaseka et al. 45, the phenol content can increase or decrease with the drying temperature. The loss of total phenolic compounds can be the result of the activity of enzymes such as polyphenol oxidase (PPO), and of its binding with proteins 44.
Table 4 shows that the flour dried at 60°C had higher antioxidant activity. These results are lower than those obtained by Alkin et al. 46, who reported ORAC values of 108.31 ± 2.91 µmol of Trolox/g dry base for Pleurotus ostreatus dried at 70°C. Tamrakar et al. 47 reported ORAC values of 945.9 ± 37.4 µmol of Trolox/g of Pleurotus ostreatus extract dehydrated at 35°C. The differences may be associated with both the drying temperature and the used extraction methods.
Table 4 Total phenol content and ORAC antioxidant capacity in Pleurotus sp flour
| Parameter | H-50 °C | H-60 °C |
|---|---|---|
| Phenols (mg GAE/g) dry basis | 3.77 ± 0.51a | 3.83 ± 0.37a |
| ORAC µmol Trolox/g dry basis | 12.93 ± 0.67b | 20.77 ± 0.20a |
Different letters in the same row indicate significant differences (p<0.05).
Source: Created by the authors.
Mutukwa 44 found a total phenolic compounds of 2.52 ± 0.19 mg GAE/g in oyster mushrooms dried by hot air at 43°C and an ORAC antioxidant activity of 11.51 ± 0.17 µmol of Trolox/g, lower than that reported in Table 4. The highest ORAC antioxidant activity found at 60°C may be related to the fact that PPO -which oxidizes phenolic compounds- is inhibited at this temperature after 10 min 48. Furthermore, polyphenols can be released from cell structures at high temperatures, thus leading to an increase in free phenolic compounds, and inducing the formation of others with better antioxidant properties 49.
Other drying technology -freeze-drying- was used for edible mushroom drying. Kho et al. 50 reported 3.54 times the total phenolic content of the oven-dried fruit bodies when using freeze drying. This may be related to greater overall phenolic extraction efficiency.
4. Conclusion
Drying with hot air at 60ºC reduced the total drying time and the final moisture content of the flour compared with drying at 50ºC, the swelling capacity and the ORAC antioxidant activity were also higher. However, the other physicochemical and functional properties did not present significant statistical differences. Therefore, hot air drying at 60ºC proved to be a suitable drying technology to obtain a mushroom powder with physicochemical and functional properties that can be used as a natural food ingredient and improve the functional properties of foods such as bakery products















