1. Introduction
COVID-19 is an infectious disease caused by the acute respiratory syndrome called Coronavirus 2 (SARS-CoV-2). The disease was first identified in December 2019 and has widely spread throughout the globe ever since, causing the COVID-19 pandemic. It has mutated over time into different variants that have infected and killed millions of people and still represent an impending threat to humanity [1]. Currently, there is no specific treatment for the virus, so an early and accurate diagnosis is crucial to make decisions on how to handle vulnerable patients and begin support-oriented therapy that can improve results, lower mortality rates, and further the success of public healthcare policies seeking to mitigate its spread [2,3].
The reverse transcription polymerase chain reaction (qRT-PCR) of the COVID-19 virus has been defined as a standard diagnosis method. The processing stage takes between 1 and 2 days, depending on the laboratory, the staff, and the sample volume. It is noteworthy to mention that there have been significant false negatives, excessive operational requirements, and high costs linked to this method [4]; however, it is still considered a mature technique with high sensitivity, which has become the gold standard for detecting the virus in its initial stage [5].
The health pandemic prompted the Federal Drug Administration (FDA) to issue emergency use authorizations for hundreds of nucleic acid molecule-based diagnostic tests and serological RT-PCR antibody tests [6]. Facilitating the commercialization of more than 350 real-time polymerase chain reaction (RT-PCR) test kits specifically designed to detect coronavirus-2019 (COVID-19) disease [7]. This context led to an inquiry on which technological alternative should be prioritized considering aspects such as sensitivity, specificity, false positives, and false negatives. The latter two can increase the risk of disease transmission and treatment delay [8], derived from the instability of the reactive elements, viral charge, and unqualified technical staff [1].
This research involves a multicriteria analysis for prioritizing PCR technologies for COVID-19 detection in Colombia, given five criteria that directly impact the decision-making process. Additionally, the tool was used to assess two technologies available in the country. This tool will help prioritize alternatives when acquiring said equipment and reduce administrative efforts for healthcare institutions in charge of COVID-19 public management.
2. Tables and figures
A bibliographical review was carried out to establish valid criteria for assessing COVID-19 diagnostic technologies. The following search equation was used: ( TITLE-ABS-KEY ( "COVID-19" ) AND TITLE-ABS-KEY ( "Health Technology Assessment" ) OR TITLE-ABS-KEY ( hta ) ) AND ( LIMIT-TO ( PUBSTAGE , "final" ) ) AND ( LIMIT-TO ( DOCTYPE , "ar" ) OR LIMIT-TO ( DOCTYPE , "re" ) OR LIMIT-TO ( DOCTYPE , "cp" ) OR LIMIT-TO ( DOCTYPE , "bk" ) OR LIMIT-TO ( DOCTYPE , "ch" ) ) AND ( LIMIT-TO ( LANGUAGE, "English" ) ) AND ( LIMIT-TO ( PUBYEAR , 2022 ) OR LIMIT-TO ( PUBYEAR , 2021 ) OR LIMIT-TO ( PUBYEAR , 2020 ) ).
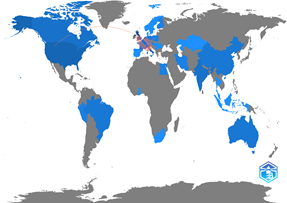
The literature review listed 59 articles published between 2020 and 2022, with an annual growth rate of 152.98%. Some research areas stand out: biochemistry, genetics, and molecular biology (47%), immunology and microbiology (8.7%), and pharmacology, toxicology, and pharmaceutics (7.7%). Nonetheless, the topic of decision-making processes was only found in 1% of the articles listed. Furthermore, the articles were primarily published in the United Kingdom (20.4%), Italy (9.3%), the Netherlands (6.5%), the United States (6.5%), and Canada (3.7%). Only Brazil (2,8%) and Peru (1,9%) have published in Latin America. Fig. 1 shows the number of articles per country in blue and the collaborations between countries in red lines.
Regarding analyzing the technology assessment criteria, the bibliographical review highlighted the work in [8-15] to characterize the selected criteria: safety, clinical effectiveness, economic aspect, organizational aspect, and legal aspect. The selection of sub-criteria involved consulting healthcare professionals with experience in RT-PCR test processing. Biomedical technology providers, technical support engineers from clinical laboratories, and healthcare staff were included to gather information on thermocyclers and technology use. The data given by the providers are kept anonymous to meet confidentiality agreements and thus will be labeled as Equipment 1 and Equipment 2.
The process was characterized for the COVID-19 RT-PCR test within the healthcare institutions encompassed by the study. The characterization allowed for the complex integration of requirements, criteria, assets, and equipment necessary for extracting and processing samples. The AHP multicriteria decision-making technique was chosen since it is widely used in different research areas such as engineering, computer science, business management, and accounting [16-20]. One of its most important characteristics is its natural ability to perform bi-univocal comparisons via matrix-based operations, allowing it to prioritize elements at one level with elements at a higher level [21].
Developing the multicriteria tool required forming a team of experts with technical knowledge to assess the standards discussed in the literature and possible PCR analysis alternatives. The results were organized into three levels of hierarchy. The goal of the assessment was set at the upper level. The criteria were established at the intermediate level. The sub-criteria and research questions were determined at the lower level. Pair-based comparisons were made of the importance of the control criteria regarding the general purpose. Additionally, a pair-based ponderation was carried out on the importance of the sub-criteria regarding each control criterion.
Once the experts had established the comparison matrices, the information was gathered in a square matrix that computed the geometric mean, defined as the nth root of the product of comparisons. Afterward, each criterion was assigned a numeric value, and its importance was quantified in a preference vector (Ni). This definition was cyclically iterated until the preference vector reached a stable state.
It was ensured that the consistency index of the paired matrices remained below 10%. The sub-criteria were established for each criterion by assessing the preference vectors of the sub-criteria and alternatives and listing the alternatives in an ascending order (where 1 represents the highest priority according to the computed value of Ck).
3. Results
Four professionals were selected from the group participating in the acquisition process and technology usage based on their education and expertise. Their profiles are described in Table 1.
The literature review identified the following assessment criteria:
Safety: Seeks to minimize patient and staff harm [23]. It includes elements regarding the use of medical devices [24].
Economic aspect: It assesses the budget-related impact for the institution that plans to acquire the equipment [24]. It includes equipment, resources, and labor costs for sample processing and analysis [25].
Organizational aspect: It encompasses the required staff, infrastructure, and training for the staff [26-28].
Clinical effectiveness: It considers accuracy-related metrics of the device, such as sensitivity, specificity, repeatability, precision, and comparison to a standard pattern. Clinical effectiveness is a critical factor in the assessment of diagnostic devices. According to the referenced sources, it is the most common criterion in HTA [26,29].
Legal aspect: The legal aspect considers the informed consent of the patient as well as their privacy and confidentiality while using the device. It also includes the legal requirements to acquire a device, the warrant policy, and the compliance with market regulations [29,30]. Processing times, maintenance, and technical support from providers are factored into this criterion [31].
Table 2 shows the five criteria and twelve sub-criteria that were most predominant according to the literature review. These were checked to be complete, non-redundant, and independent.
Table 1 Profile of the decision-making group
| Expert | Description |
|---|---|
| 1 | Administrative Director of a Clinic in the savannah of Cundinamarca |
| 2 | Purchasing manager of a clinic in the savannah of Cundinamarca |
| 3 | Biomedical Engineer who plays this role in the hospital environment for a Clinic in the Savannah of Cundinamarca |
| 4 | Bacteriologist with PCR test processing experience |
Source: The Authors.
Table2. Decision criteria and sub-criteria
| Criteria | Sub-criteria | Definition |
|---|---|---|
| Safety | Risk associated with healthcare personnel | Hazards to which care personnel are exposed during the process [32] |
| Associated adverse events | Damage associated with care personnel, operator, or the environment because of the use of the equipment [33] | |
| False positives and negatives | The probability of getting a positive test in a patient who does not have the disease or the probability of having a test result is negative when the patient has the disease.[34], [35] | |
| Economic | Cost of equipment | Global price of the equipment in the market, in this case, the price the manufacturer assigns when it is launched on the market, including its after-sales services. |
| Resources and inputs | Personnel needed to operate the equipment and what supplies it needs to function correctly. | |
| Organizational | Infrastructure | Physical requirements for the operation of the equipment, including modifications and adaptations required for the location of the equipment, in addition to the requirements in infrastructures proposed by ISO 15189 2022.[31] |
| Personnel and training | Bacteriologists in charge of processing Covid-19 tests. Training is provided by suppliers to staff. | |
| Quality control |
|
|
| Clinical effectiveness | Measurement | Evaluates sensitivity, specificity, effectiveness, and accuracy [37] |
| Legal Technician | Technical service | The after-sales service provided by the supplier corresponds to preventive and corrective maintenance that the equipment needs during the loan or sale, according to the negotiation in the tender. It also includes the automatic fault solutions that the equipment has and the modality of provision of the technical service. |
| Times | Refers to the supplier's response times to equipment failure to perform corrective maintenance. It also considers the test processing times the team uses. | |
| Characteristics | Global technical characteristics of the equipment such as number of simultaneous tests it can process, what type of tests it processes, software and interfaces for communication and transmission of information, and its useful life. |
Source: The Authors.
Table 3 Preference vector
| Criteria | Preference vector of criteria | Sub-Criteria | Preference vector of sub-criteria |
|---|---|---|---|
| Clinical effectiveness | 32.7% | Measurement | 100% |
| Safety | 29.8% | Adverse events | 49% |
| Risk associated with healthcare personnel | 35.7% | ||
| False positives and negatives | 15.3% | ||
| Economic | 16.4% | Cost of equipment | 50% |
| Resources and inputs | 50% | ||
| Legal Technician | 10.8% | Technical service | 36.4% |
| Times | 29% | ||
| Characteristics | 34.6% | ||
| Organizational | 10.3% | Infrastructure | 28.7% |
| Personnel and training | 22.1% | ||
| Quality control | 49.1% |
Source: The Authors.
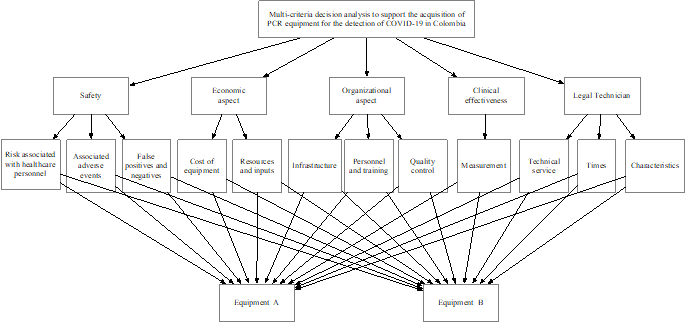
The hierarchical structure is presented in Fig. 2, including the assessment's equipment, criteria, and sub-criteria. This structure is based on the work of Saaty [38,39].
The expert responses were collected in Table 3 with a consistency index 0.077.
The results of the preference vector were used to assess the two medical equipment in Table 4. Equipment 2 prioritized the safety criteria with 53.16%, while Equipment 1 scored 46.18%.
Table 4 Results of the assessment
| Criteria | Equipment 1 | Equipment 2 |
|---|---|---|
| Clinical effectiveness | 50% | 50% |
| Safety | 46.18% | 53.82% |
| Economic | 54.17% | 45.83% |
| Legal Technician | 32.25% | 67.75 |
| Organizational | 50% | 50% |
Source: The Authors
The clinical effectiveness and organizational criteria showed similar features for both equipment and had the same weight in the assessment. Equipment 1 obtained an economic criterion of 54.17% compared to 45.83% for Equipment 2, given some differences in pricing and post-sale services that improved the overall product. The legal aspect marks a significant advantage for Equipment 2, scoring 67.75% compared to 32.25% for Equipment 1; this was primarily due to certain features, such as the useful life of the equipment.
After the criteria and sub-criteria percentages of the expert responses were tallied, both equipment were assessed with the tool leading to the preference vector in Table 5. Equipment 2 is slightly better than Equipment 1.
4. Discussion
Businesses targeting in-vitro diagnostic methods have increasingly automated equipment that meets the features analyze in this study [40]; however, economic limitations or even infrastructure conditions are needed for molecular testing, given the sample volumes and types of patients handled by healthcare institutions.
The methods described in this work enable an assertive selection of the technology required by healthcare institutions while also meeting the intrinsic needs of the service. It is essential to remember that respiratory illnesses' symptoms are similar, and in vitro molecular diagnostics methods can identify the agents causing the illness at an early stage. Real-time PCR not only allows the differentiation of causal agents (fungi, bacteria, viruses, or parasites) but also contributes to offering timely and effective treatment to the patients, which increases the control of antibiotic resistance, pandemic status control, and the reduction of hospital occupation and healthcare costs.
Different tests can support patient care and disease control, specifically regarding COVID-19; some tests can directly detect the virus (antibody detection or PCR), while others can detect the immune response to the virus (antibodies). According to SARS-CoV-2 guidelines issued by the Health Ministry, PCR tests are official laboratory confirmations of COVID-19 since they are based on the detection of genetic sequences of the virus (RNA). These also have high sensitivity and specificity and are approved by the PAHO/WHO to confirm the diagnosis of the disease [41].
Real-time PCR tests are based on obtaining complementary DNA (cDNA) from an RNA chain through reverse transcription (RT). Subsequently, small viral genome sequences are detected using real-time PCR, seen as the gold standard in diagnosis and epidemiologic tracking of the disease. Nonetheless, the incubation period plays a significant role in the detection process, given that the presence of the virus is less likely during the first five days of the illness. A successful and effective patient diagnosis is linked to different variables: exposure time, presence and severity of the symptoms, viral load present in the blood, sample quality and origin (lower or upper respiratory tract), type, class, and testing method. This method requires approximately 1 hour for extraction and 3 hours for amplification, with a window of opportunity of 1 day. It also allows the simultaneous processing of various samples.
Multiplex PCR is another standard detection method that simultaneously identifies pathogens causing respiratory infections in less than 1 hour without requiring manual or mechanical extraction. This rapid detection reduces the chance of contamination and variability in the processes of the staff in charge of the test. These are highly sensitive and specific while also being approved by health regulators. In an hour, one patient can be processed [42].
The research implemented a multicriteria tool to support acquiring PCR equipment to detect COVID-19 in Colombia. This tool can be applied in acquiring medical equipment of these characteristics in other countries. The criteria focused on safety and clinical effectiveness compared with other relevant economics, legal technician, and organizational aspects.
Regarding clinical effectiveness, the questions aim to obtain precise and detailed information on the characteristics of the equipment, such as sensitivity, specificity, and precision, with the aim of understanding and evaluating performance in terms of its ability to detect and measure precisely critical aspects of the equipment. It will end that clinical effectiveness plays a fundamental role and has a higher impact on other assessment criteria. It is essential to diagnose in the shortest possible time to define appropriate behavior and achieve an effective, efficient, and timely improvement in the patient.
The safety criteria focused on obtaining relevant information on the risks associated with using the equipment, knowing if the healthcare personnel have experienced injuries during its use, and what those injuries have been. In addition, to obtain data on adverse events reported using the medical equipment. Finally, information on the percentage of false positives and false negatives related to the personnel and the test processing was considered.
The technician legal and organizational criteria present similar weightings (10.8% and 10.3%) attributable to the standardization of these criteria in the market under sub-criterion such as customer service, hourly availability for telephone or virtual attention, total processing time, number of simultaneous tests that the equipment can process and whether it is capable of processing tests of a different type than those of Covid-19 and useful life of the equipment in years. Regarding the organizational criteria, sub-criteria are evaluated, such as relevant information on the sales process, training, and quality control following quality standards, availability and flexibility of training, and device quality assurance.
The tool developed in this research is made available to any institution interested in acquiring PCR-test equipment for the detection of COVID-19. The results presented in this work are restricted by the information given by the technology providers.
5. Conclusions
The success of the RT-PCR mainly depends on the pre-and post-analytical variables of the sample processing. Many factors can lead to false negatives, such as deterioration of the genetic material and insufficient extraction. Test results are influenced by various conditions: adequate uptake, transportation, preservation of the samples, storage, and preservation of reactive components, and the training and expertise of the staff in charge.
The proposed model implemented a systematic approach based on hierarchical analysis to evaluate in a multicriteria way the acquisition of PCR tests for detecting COVID-19 in a health institution. The objective was to solve the general problem by identifying the relevant criteria and sub-criteria, which were evaluated using technical information, data provided by health organizations, and statements from the representatives of the brands in Colombia.
This methodology made it possible to identify the best alternative through mathematical tools, which contributed to reducing the subjectivity and uncertainty associated with the decision-making process. By applying a multicriteria approach, it was possible to recognize and work within the framework of the existing organizational culture, which favored greater objectivity in the evaluation and selection of PCR equipment.
The sample's clinical effectiveness and processing time significantly impact the assessment of the medical equipment and the in vitro diagnostic reactive agents. In contrast, the legal and organizational aspects are considered secondary.