Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418On-line version ISSN 1909-6356
Rev. colomb. cienc. quim. farm. vol.37 no.2 Bogotá July/Dec. 2008
Artículo de investigación
Energía de Gibbs para los procesos de transferencia del triclosan desde el agua hasta algunos solventes orgánicos a 25,0 °C
Gibbs energy of transfer processes for the antimicrobial agent Triclosan from water to some organic solvents at 25.0 °C
Diana M. Aragón1, Diego A. Chiappetta2,3, José Degrossi 4, Edgar F. Vargas5, Carlos Bregni2, Alejandro Sosnik2,3, and Fleming Martínez1
1 Department of Pharmacy, Faculty of Sciences, National University of Colombia, Bogotá D.C., Colombia.
2 Department of Pharmaceutical Technology, Faculty of Pharmacy and Biochemistry, University of Buenos Aires, Buenos Aires, Argentina.
3 National Science Research Council (CONICET), Buenos Aires, Argentina.
4 Department of Toxicology, Faculty of Pharmacy and Biochemistry, University of Buenos Aires, Buenos Aires, Argentina.
5 Department of Chemistry, Faculty of Sciences, Los Andes University, Bogotá D.C., Colombia.
* Corresponding author: Fleming Martínez.
Recibido para evaluación: septiembre 1 de 2008 Aceptado para publicación: noviembre 10 de 2008
RESUMEN
En este trabajo se presentan las energías de Gibbs para los procesos de disolución del triclosan (TS) en solventes orgánicos de diferente capacidad de formación de enlace de hidrógeno, las cuales fueron calculadas a partir de los valores de solubilidad a 25,0 °C. La solubilidad del TS se determinó en etanol, octanol, octanol saturado de agua, miristato de isopropilo, cloroformo, y heptano. Así mismo se calcularon las energías de Gibbs de exceso y los coeficientes de actividad del soluto en los mismos solventes. Adicionalmente, mediante el uso de valores previamente reportados en la literatura, se calcularon las energías de Gibbs de transferencia del TS desde el agua hasta los solventes orgánicos comprendidos en el estudio. En todos los casos, esta propiedad termodinámica fue negativa demostrando la preferencia del TS por los medios orgánicos evaluados.
Palabras clave: Triclosan, solubilidad, transferencia, termodinámica de soluciones, solventes orgánicos.
ABSTRACT
The thermodynamic function Gibbs energy for the dissolution processes of triclosan (TS) was calculated from solubility values obtained at 25.0 °C in organic solvents with different hydrogen-bonding capability. TS solubility was determined in ethanol, octanol, water-saturated octanol, isopropyl myristate, chloroform, and heptane. The excess Gibbs energy and the activity coefficients of the solute were also calculated. In addition, the corresponding Gibbs energies of the drug transfer process from water to the organic solvents under investigation were also calculated by means of previous reports. In all cases, this thermodynamic property comprised a negative value, indicating the preference of TS for all the organic media evaluated.
Key words: Triclosan, solubility, transfer, solution thermodynamics, organic solvents.
INTRODUCTION
Triclosan (5-chloro-2-(2,4-dichlorophenoxi)-phenol, TS), is a potent synthetic bactericide and fungicide with notably high chemical stability and persistent activity (1). The accepted mechanism of action is the diffusion across the cytoplasm and the inhibition of the synthesis of RNA, lipids and proteins (2). Other studies, suggested that TS effectively inhibits enzymes involved in the metabolism of lipids of Escherichia coli (3,4). Due to these features, TS has been extensively used through the years in a diversity of topical applications (2). In 1997 it was approved by the FDA for use in oral care products such as toothpastes (Colgate® Total) and its application gained even more impact with the development of mouthwashes and other formulations for plaque prevention and control of periodontal disease (5-7). More recently, Ethicon Inc. has introduced poly(lactic-glycolic acid) biodegradable sutures coated with TS (Vicryl®) (8).
One important limitation in the development of TS-loaded topical products is the po or aqueous solubility of the drug (9). This behavior stems from the high hydrophobicity of the molecule. On the other hand, the presence of one aromatic -OH group that is ionizable at pH > 10 enables better solubilization under alkaline pH conditions. However, such alkalinity is incompatible with medical applications. Several approaches were investigated in order to improve the solubility of TS in neutral aqueous media. Lofftson et al. designed complexes with β-cyclodextrins (10-12). Grove and co-workers investigated other molecular complexes, micelles and the in situ formation of organic salts (13). Maestrelli, García-Fuentes, Mura & Alonso (14) developed chitosan- hydropropylcyclodextrin nanocarriers and investigated the water-solubilization of TS. Findings showed a 20-fold increase in the solubility of the drug. In another work, Friedman and collaborators reported on the development of ethylcellulose TS-containing buccal patches for sustained release of the drug. The device effectively released TS following a Higuchi model and affected the viability of Streptococcus mutans, a frequent pathogen in periodontal disease (15).
More recently, we evaluated the solubilization of TS by means of inclusion into poloxamine (a four-arm poly(ethylene oxide)-poly(propylene oxide) block copolymer) polymeric micelles in a broad range of pH values and polymer concentrations (16,17). Solubility values increase up to 4 orders of magnitude. Moreover, the hydrogen bonding ability played a central role in the drug-nanocarrier interaction. Thus, ionic TS (at pH ˜12) displayed a weaker affinity by the micelles and this phenomenon rendered lower solubilization extents (16,17). More importantly, TS-loaded systems showed antibacterial activity in-vitro against a broad spectrum of pathogens, including two representative clinical pathogens: methicilin-resistant Staphylococcus aureus and vancomycin- resistant Enterococcus faecalis. Finally, the activity was assessed on biofilms of Staphylococcus epidermidis a bacteria highly recurrent in Biomaterial Centered Infections (BCI), with encouraging results (17).
The profuse use of this agent has raised important environmental concerns due to its accumulation in wastewater streams (18,19). As in the case of pre-formulation and formulation process of pharmaceutical preparations, toxicity to aquatic life and appearance in drinking water is directly related to solubility in water.
Surprisingly, the fundamental aspects of the dissolution process (e.g., thermodynamic functions) in solvents like water and organic media were not thoroughly investigated. In this context, the present work studied the TS solubility thermodynamics in six different organic model solvent systems used for Quantitative Structure-Activity Relationships (QSAR) studies. The goal of the present research was to present a more complete and systematic insight on the properties of dissolution and transfer for this drug. The solubility at 25.0°C was determined in ethanol, octanol, water-saturated octanol, isopropyl myristate, chloroform and heptane, and the respective dissolution thermodynamic analysis was made by using the Gibbs equation. Octanol has been used as standard organic medium for partition experiments in the development of QSAR studies, because the octanol-water partition coefficient (log P) is an important parameter for modeling biological membranes and predicting the fate, transport and distribution of drugs (20). Isopropyl myristate is best related to skin/transdermal absorption because its polar and non-polar nature mimics the complex nature (semipolar matrix) of the skin (21,22). Chloroform is an organic solvent acting mainly as hydrogen donor in establishing hydrogen bonds. Heptane is a lipophilic hydrocarbon solvent, purely non-polar, interacting by London forces, enabling the evaluation of solute- solvent non-specific interactions (23). Finally, the contribution due to the mixingprocess toward the overall dissolution was also analyzed by using the values reported for the TS fusion process by Veiga, Merino, Cirri, Maestrelli & Mura (24).
EXPERIMENTAL
Materials
Triclosan was a kind gift of Ciba C.S. (Bogotá, Colombia); octanol (ROH) extra pure, Merck; isopropyl myristate (IPM) F.S., Merck; chloroform (CLF) A.R. Mallinckrodt; heptane (HPT) F.A., Merck; absolute ethanol (EtOH) A.R., Merck; distilled water, conductivity < 2 µS cm-1 from Laboratory of Pharmaceutics; Millipore Corp. Millex®-13mm filters.
Solubility determinations
TS was added in excess to the corresponding organic solvent (20 cm3). The solid-liquid mixtures were then stirred in a mechanical shaker (Wrist Action, Burrel, model 75), for 1 h. Samples were then allowed to stand in water baths (Magni Whirl Blue M. Electric Company) kept at 25.0 ± 0.05 °C for at least 5 days to reach the equilibrium (this equilibrium time was established by quantifying the drug concentration until a constant value was obtained). Once at equilibrium, supernatant solutions were filtered (at isothermal conditions) in order to remove insoluble particles before analysis. Drug concentrations in ROH, ROH(W), and IPM were determined by measuring absorbance after appropriate dilution and interpolation in previously constructed UV calibration curves for TS in absolute ethanol (UV/VIS BioMate 3 Thermo Electron Company). On the other hand, the drug concentrations in EtOH, CLF and HPT were determined by mass balance by weighting a specified quantity of the respective saturated solution and allowing the solvent evaporation up to constant mass. In order to allow the conversion between concentration scales, the density of the saturated solutions was determined with a digital density meter (DMA 45 Anton Paar, precision ± 0.0001 g cm-3). All experiments were made at least three times and averaged.
RESULTS AND DISCUSSION
In Table 1, the molecular structure of TS and some of their physicochemical properties are summarized (25). This drug acts in solution mainly as a Lewis acid (phenolic OH group) in order to establish hydrogen bonds with proton-acceptor functional groups present in the solvents (oxygen in -OH and >C=O groups).
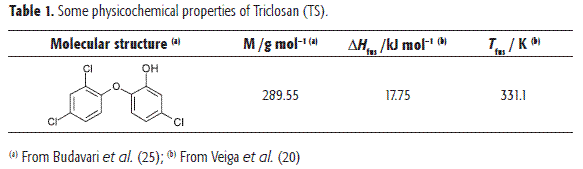
Ideal and experimental solubility of TS
The ideal solubility of a crystalline solute in a liquid solvent can be calculated by Eq. [1]:
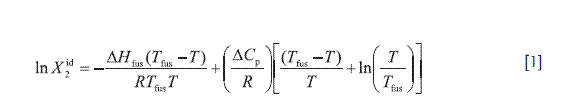
Where is the ideal solubility of the solute as mole fraction, ΔHfus is the molar enthalpy of fusion of the pure solute (at the melting point), Tfus is the absolute melting point, T is the absolute solution temperature, R is the gas constant (8.314 J mol-1 K-1), and ΔCp is the difference between the molar heat capacity of the crystalline form and the molar heat capacity of the hypothetical supercooled liquid form, both at the solution temperature (26). Since the experimental determination of ΔCp is difficult, its value is usually approximated to the entropy of fusion, ΔSfus.
Table 2 summarizes the experimental solubilities of TS, expressed in molarity and mole fraction, in addition to the ideal solubility calculated by means of Eq. [1] from ΔHfus, and Tfus presented in Table 1. In almost all the cases, the coefficients of variation for experimental solubility were smaller than 2.0%. The aqueous value was taken from Loftsson and Hreinsdóttir (27).
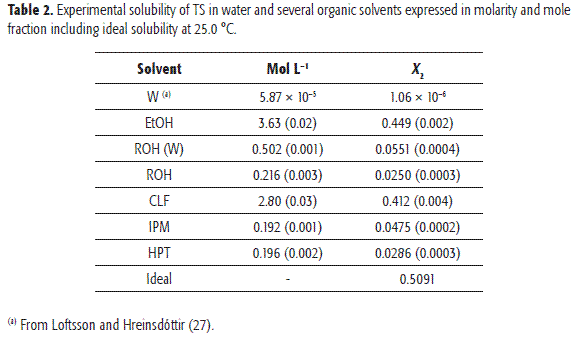
Regarding the different organic solvents, the highest solubility value (in mole fraction) was observed in pure EtOH, while the lowest value was found in ROH. In addition, solubility values gradually decreased following the order EtOH > CLF > ROH(W) > IPM > HPT > ROH. However, no reports on solubility values of TS in the solvents here investigated are available, and therefore no direct comparison is possible. This behavior is similar to those found for other compounds, namely, some analgesic drugs (28-30)
TS solubility analysis in terms of solubility parameters
Although dissolution is a complex phenomenon, intents have been proposed in order to explain this important physicochemical property of drugs. One of them was proposed by Hildebrand, Prausnitz & Scott (31) in terms of the solubility parameter, δ, which is defined as the root square of cohesive energy density, and it is calculated according to Equation [2]:

Where, ΔHvap is the vaporization enthalpy and V is the molar volume. Hildebrand solubility parameters were initially proposed for nonpolar compounds interacting among them by dispersion forces (London forces); nevertheless, TS and almost all the solvents investigated interact by London forces and also by other more energetic forces, namely, dipolar forces and hydrogen-bonding. In this context, Hansen split the general δ values in three partial parameters considering the respective contributions by dispersive forces δd, dipolar forces δp, and hydrogen-bonding δh (32). These subparameters are related to total solubility parameter δT, according to:
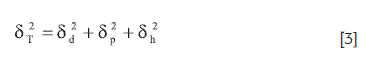
The experimental determination of partial solubility parameters is not easy and therefore some calculus methods based on the contribution of groups have been described. The methods more used are those proposed by Fedors and van Krevelen, and described by Hansen (33). In this context, Table 3 summarizes the Fedors and van Krevelen analysis for TS. It is worth noting that the values presented are not coincident with those presented by Hansen (34). Discrepancies could stem from the different values assigned to each group in the respective analyses. Nevertheless, it can be seen in Table 3 that London forces are the most relevant for this compound, which could be attributed mainly to chlorine atoms and aromatic rings. Thus, based on the δT value (29.4 MPa1/2), TS could be considered as semipolar compound.
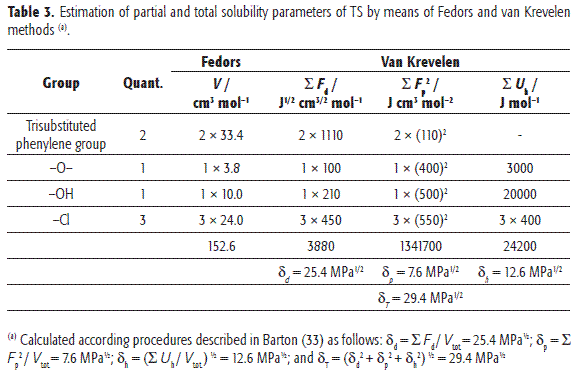
On the other hand, according to Martin and Bustamante (35), the greatest drug solubility value should be found in solvents with similar δ values. For this reason, Table 4 summarizes these values for the organic solvents tested, as well as for water (33,36). It is necessary to note that the values for ROH(W) were calculated from the respective values for ROH and W, as pure solvents, by considering them as additive properties, based on volume fractions (37). The equilibrium composition of water-saturated octanol at 25.0 °C was taken from Dallos and Liszi (38).
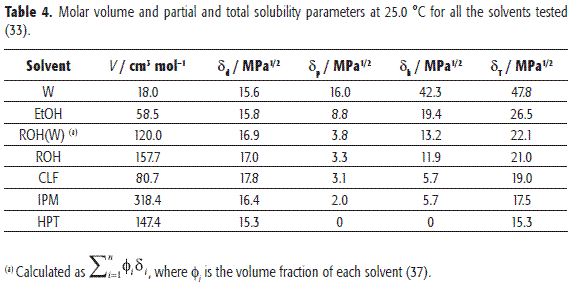
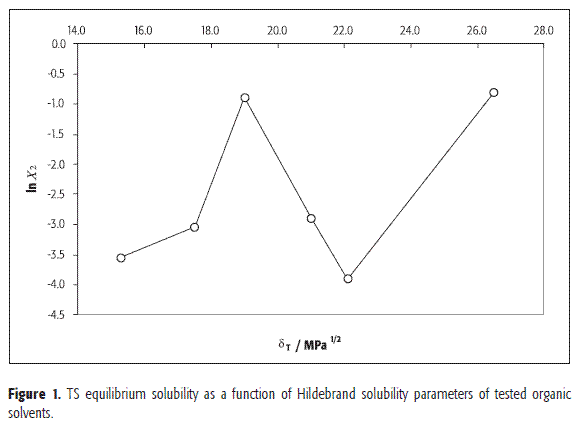
Apparently, no similarity in all δ values is observed by comparing TS (Table 3) in all the solvents tested (Table 4) when they are related to the equilibrium solubilities (Table 2). This fact demonstrates that the solubility of a certain drug compound is a more complex phenomenon than that exclusively described by solubility parameters and without considering other properties. In the same way, Figure 1 clearly shows that no simple relation between TS equilibrium solubility and solvents δ values is found.
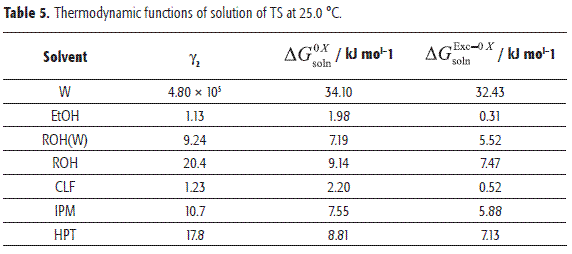
TS activity coefficients
The solute activity coefficient in the solution (γ2) is calculated as Xid2/X2and it is an indication of the deviation presented by TS from its ideal behavior (26). Table 5 shows the TS activity coefficients.
From the γ2 values an approximate estimation of solute-solvent intermolecular interactions can be made by considering the following expression:
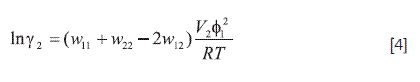
where w11, w22 y w12 represent the solvent-solvent, solute-solute and solvent-solute interaction energies, respectively; V2 is the molar volume of the supercooled liquid solute, and finally, Φ1 is the volume fraction of the solvent. In a first approach the term (V2Φ12 /RT)T,P may be considered approximately constant at the same temperature, and then γ2 depends almost exclusively on w11, w22 and w12 (39). While the term w12 favors the solution process, both w11 and w22 terms are unfavorable for solubility. The contribution of w22 represents the work necessary to transfer drug molecules from the solid to the vapor state and, therefore, it is constant in all organic solvents. The solution in pure IPM having a γ2 value around 10 implies a relatively low w12 value. In EtOH and CLF solutions having γ2 values near to 1 and w11 values relatively low, then the w12 value would be large. On the other hand, in ROH, ROH(W), and HPT solutions, having γ2 values around 20, the analysis based on w12 and/or w12 terms is less clear and so neither solvent-solvent or solute-solvent contributions could be inferred.
Solution Gibbs energy of TS
The solubility allows the calculation of the standard Gibbs energy of transfer of the solute from its own phase to a saturated solution (40). If the mole fraction scale is used, the standard Gibbs energy of solution (ΔG0soln) of non electrolytes, such as TS, may be calculated by means of Equation [5].

According to Table 3, it is found that is positive in all cases; i.e., the solution process apparently is not spontaneous, which may be explained in terms of the concentration scale used (mole fraction), where the reference state is the ideal solution having the unity as the concentration of the drug under investigation; this represents the solid pure solute.
Solution excess Gibbs energy of TS
The solution process may be represented by the following hypothetic stages (40):
Solute(Solid) → Solute(Liquid) → Solute(Solution)
Where, the respective partial processes toward the solution are solute fusion (related to drug ideal solubility) and mixing (related to the drug non-ideal solubility or excess solubility) at the same temperature (25.0 °C), which enables to calculate the partial thermodynamic contributions to the overall solution process by means of Equation [6].
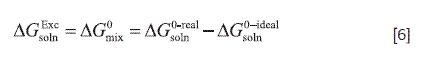
According to Table 3 it is apparent that is positive in all cases, which may be explained in terms of the lower experimental TS solubility in comparison to the ideal solubility.
Transfer Gibbs energy of TS from water to organic solvents
In order to contribute with the generation and systematization of the thermodynamic quantities of transfer useful in QSAR studies, values for the transfer of TS from water to organic solvents were determined. In Table 6 the Gibbs energy of transfer are shown. The thermodynamic quantities were calculated as the difference between the solution functions in organic solvents (Table 5) and those for aqueous media showed also in Table 5 and based on a solubility value presented by Loftsson and Hreinsdóttir (27), according to Equation [7]. At neutral pH the molecular form of TS without dissociation predominates and thus, the transfer of the non-dissociate drug should be considered.
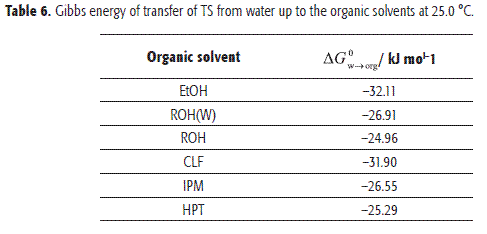

Accordingly to Table 6 the transfer process of this drug from water to all organic solvents is spontaneous (ΔG0W→org<0), indicating the preference of TS for organic media.
In the partitioning process of the drug between organic solvents and water, the enthalpic and entropic changes are also important and imply respectively, the energetic requirements and the molecular randomness (increase or decrease in the molecular disorder), implied in the net transfer of the drug from water to different organic media. In general terms, the behavior presented in each phase, before and after the partitioning process, should be considered independently. Since initially the solute is present only in water, then, it is necessary to create a cavity in the organic medium in order to accommodate the solute after the transfer process. This is an endothermic event, since an energy supply is necessary to separate the organic solvent molecules. When the solute molecules are accommodated in the organic phase, an amount of energy is released due to the generation of solute-organic solvent interactions. This event implies an entropy increase in this organic medium due to the mixing process. On the other hand, the original cavities occupied by the drug in the aqueous phase have been now occupied by water molecules; this phenomenon taking place after a certain number of solute molecules have migrated from the aqueous to the organic phase until equilibrium is reached . This event produces an energy release due to the formation of water-water interactions. However, depending on the moieties present in the molecular structure of the drug, it is also necessary to consider the possible disruption of the water structure, that is, the water molecules organized around the alkyl or aromatic groups (hydrophobic hydration). This event in particular implies an intake of energy, in addition to a local entropy increase by the separation of some water molecules which originally were associated among them by hydrogen bonding (41). Hence, it would very important to determine experimentally the previously discussed thermodynamic quantities of transfer, by means of the partition coefficients determination, in order to confront the apparent values presented in Table 6. It is also necessary to take into consideration here that the partitioning experiments are carried out at low drug concentrations where the solute-solute interactions are not present (24); whereas, in the solubility analysis these interactions would be present in the organic solvents, due to relatively high the solubility values, and therefore, the thermodynamic quantities also include these interactions, in addition to solute-solvent ones. Based on partition coefficients the Gibbs energy of transfer (ΔG0W→org) is calculated by means of Equation [8]:
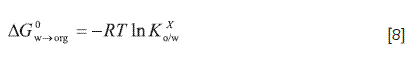
In which, the partition coefficient (Kxo/w) is expressed in mole fraction, which is thus calculated from the value expressed in molarity (Kco/w) by means of Equation [9]:

Where, Vo and Vw are the molar volumes of the organic and aqueous phases, (120.0 cm3 mol-1 and 18.0 cm3 mol-1), respectively. This equation is valid because the fact that the drug solutions in both media are highly diluted; thus, the Vo and Vw values are almost coincident with those presented for the pure mutually saturated solvents.
According to this and considering that log Kco/w is 4.76 (27), and therefore, Kco/w= 57544, it follows that Kxo/w is 381689. Then, ΔG0W→org is -31.86 kJ mol-1. This value is different than the -26.91 kJ mol-1 presented in Table 6 (corresponding to the transfer of TS from drug saturated-water to drug saturated-water saturated octanol and obtained by studying solubilities). As previously depicted, this difference could be attributed to solute-solute interactions in the saturated organic phase.Dilution Gibbs energy of TS in ROH(W) medium
Another interesting process is the drug dilution in the organic solvents from saturation to dilute solutions (42). The respective thermodynamic function (ΔG0dilution) is calculated according to:
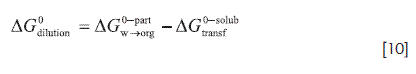
where, ΔG0-partW→org and ΔG0-solubtransf as indicated earlier, are the Gibbs energy of transfer from aqueous to organic media obtained from partitioning and dissolution processes, (-31.86 kJ mol-1 and -26.91 kJ mol-1), respectively. Thus ΔG0dilutionin ROH(W) is -4.95 kJ mol-1. The dilution process essentially implies the diminishing solute-solute interactions and concomitantly increasing solute-solvent interactions as well as the solvent-solvent interactions. According to ΔG0dilution the value, the dilution process is spontaneous. Otherwise, since energy involved in the process must be supplied in order to overcome the solute-solute interactions during the dilution process, hence, the drug partial enthalpy and entropy increase as well. Also, the increase in the solventsolvent interactions caused by the drug dilution process implies either a decrease in the solvent partial enthalpy and entropy. Enthalpic and entropic terms of Gibbs energy of dilution are required in order to propose some molecular explanation to this finding. It is worth considering that in the literature a possible organization based on a number of centers conformed by two water molecules and six octanol molecules, inside the microheterogeneous structure of this water-saturated organic solvent has been proposed that (21,43). The thermodynamic values for the dilution process correspond to the net result obtained by considering the partial contributions of solutesolute and solvent-solvent interactions, as well. Nevertheless, in order to clarify and understand the specific interactions presented between this drug and all the organic solvents studied, it would be very important to dispose information about UV, IR and NMR spectral data, and DSC and dissolution calorimetric values, among others.
CONCLUSIONS
From the previously exposed analysis, in general terms it could be concluded that TS has mainly a lipophilic behavior but in turn it is certainly not a hydrophobic drug. Otherwise, apparently the hydrogen bonding plays an important role in the drug transfer process from aqueous media up to the organic solvents based on the Gibbs energies obtained. Finally, it could be said that physicochemical values reported here would be useful for understanding the pharmaceutical behavior and performance of this drug in modern dosage forms recently developed (44).
ACKNOWLEDGMENTS
We thank Ciba C.S. Colombia for donating us the triclosan and the DIB-DINAIN of the National University of Colombia (NUC) for the financial support. Additionally we thank the Department of Pharmacy of NUC for facilitating the equipment and laboratories used.
REFERENCES
1. H.N. Bhargava, P.A. Leonard, Triclosan: Applications and safety, Am. J. Infect. Control., 24 209-218 (1996). [ Links ]
2. R.D. Jones, H.B. Jampani, J.L Newman, A.S. Lee, Triclosan: A review of effectiveness and safety in health care settings, Am. J. Infect. Control., 28, 184-196 (2000). [ Links ]
3. C.W. Levy, A. Roujeinikova, S. Sedelnikova, P.J. Baker, A.R. Stuitje, A.R. Slabas, D.W. Rice, J.B. Rafferty, Molecular basis of triclosan activity, Nature, 398, 383- 384 (1999). [ Links ]
4. F. Fan, Defining and combating the mechanisms of triclosan resistance in clinical isolates of Staphylococcus aureus. Antimicrobial Agents & Chemotherapy, 46, 3343-3347 (2002). [ Links ]
5. Food and Drug Administration, FDA approves first toothpaste for gum disease, FDA Talk paper, July 14th (1997). [ Links ]
6. M.L. Barnett, The role of therapeutic antimicrobial mouthrinses in clinical practice: Control of supragingival plaque and gingivitis, J. Am. Dental Assoc., 134, 699-704+741(2003). [ Links ]
7. K.P.K.J. Hioe, G.A. van der Weijden, The effectiveness of self-performed mechanical plaque control with triclosan containing dentifrices, Int. J. Dental Hygiene, 3, 192-204(2005). [ Links ]
8. M. Storch, H. Scalzo, S., Van Lue, G. Jacinto, Physical and functional comparison of coated Vicryl Plus antibacterial suture (coated polyglactin 910 suture with tricloscan) with Vicryl suture (coated polyglactin 910 suture), Surgical Infections, 3, Issue Suppl. 1, S-65-S-77 (2002). [ Links ]
9. T. Loftsson, N. Leeves, B. Bjornsdottir, L. Duffy, M. Masson, Effect of cyclodextrins and polymers on triclosan availability and substantivity in toothpastes in vivo, J. Pharm. Sci., 88, 1254-1258 (1999). [ Links ]
10. J. Lu, M.A. Hill, M. Hood, D.F. Greeson Jr., J.R. Horton, P.E. Orndoff, A.S. Herndon, A.E. Tonelli, Formation of antibiotic, biodegradable polymers by processing with Irgasan DP300R (Triclosan) and its inclusion compound with B-cyclodextrin, J. Appl. Polym. Sci., 82, 300-309 (2001). [ Links ]
11. T. Loftsson, í.B. Össurardótti, T. Thorsteinsson, M. Duan, M. Másson, Cyclodextrin solubilization of the antibacterial agents´ triclosan and triclocarban: Effect of ionization and polymers, J. Incl. Phenom., 52, 109-117 (2005). [ Links ]
12. M.S. Duan, N. Zhao, í.B. Össurardóttir, T. Thorsteinsson, T. Loftsson, Cyclodextrin solubilization of the antibacterial agents triclosan and triclocarban: Formation of aggregates and higher-order complexes, Int. J. Pharm., 297, 213-222 (2005). [ Links ]
13. C. Grove, W. Liebenberg, J.L. Du Preez, W.Yang, M.M. De Villiers, Improving the aqueous solubility of triclosan by solubilization, complexation, and in situ salt formation, J. Cosmetic Sci., 54, 537-550 (2003). [ Links ]
14. F. Maestrelli, M. García-Fuentes, P. Mura, M.J. Alonso, A new drug nanocarrier of chitosan and hydroxypropylcyclodextrin, Eur. J. Pharm. Biopharm., 63, 79-86 (2006). [ Links ]
15. D. Steinberg, T. Tal, M. Friedman, Sustained-release delivery systems of triclosan for treatment of Streptococcus mutans biofilm, J. Biomed. Mater. Res., 77B, 282-286 (2006). [ Links ]
16. D.A. Chiappetta, J. Degrossi, S. Teves, E.F. Vargas, D.M. Aragón, F. Martínez, A. Sosnik, Nanotechnological strategies for the treatment of bacterial biofilm, II National Symposium on Nanotechnology: NanoForum Colombia 2007, Bogotá D.C., Colombia, 2007. [ Links ]
17. D.A. Chiappetta, J. Degrossi, S. Teves, M. D´Aquino, C. Bregni, A. Sosnik, Triclosan- loaded poloxamine micelles for enhanced antibacterial activity against biofilm, Eur. J. Pharm. Biopharm., 69, 535-545 (2008). [ Links ]
18. D.C. McAvoy, B. Schatowitz, M. Jacob, A. Hauk, W.S. Eckhoff, Measurement of triclosan in wastewater treatment systems, Environ. Toxicol. Chem., 21, 1323- 1329 (2002). [ Links ]
19. D.R. Orvos, D.J. Versteeg, J. Inauen, M. Capdevielle, A. Rothenstein, V. Cunningham, Aquatic toxicity of triclosan, Environ. Toxicol. Chem., 21, 1338-1349 (2002). [ Links ]
20. J. Sangster, "Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry", John Wiley & Sons, Chichester, 1997. pp. 1-55. [ Links ]
21. K.B. Sloan, S.A.M. Koch, K.G. Siver, F.P. Flowers, Use of solubility parameters of drug and vehicle to predict flux thorough skin, J. Invest. Dermatol., 87, 244-252 (1986). [ Links ]
22. M.H. Abraham, W.E. Acree Jr., Characterisation of the water-isopropyl myristate system, Int. J. Pharm., 294, 121-128 (2005). [ Links ]
23. C.P. Mora, F. Martínez, Thermodynamic study of partitioning and solvation of (+)-naproxen in some organic solvent/buffer and liposome systems, J. Chem. Eng. Data 52, 1933-1940 (2007). [ Links ]
24. M.D. Veiga, M. Merino, M. Cirri, F. Maestrelli, P. Mura, Comparative study on triclosan interactions in solution and in the solid state with natural and chemically modified cyclodextrins, J. Inclusion Phenomena Macrocyclic Chem., 53, 77-83 (2005). [ Links ]
25. S. Budavari, M.J. O´Neil, A. Smith, P.E. Heckelman, J.R. Obenchain Jr., J.A.R. Gallipeau, M.A. D´Arecea, "The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals", 13th ed., Merck & Co., Inc., Whitehouse Station, NJ, 2001. [ Links ]
26. D.P Pacheco, Y.J. Manrique, F. Martínez, Thermodynamic study of the solubility of ibuprofen and naproxen in some ethanol + propylene glycol mixtures, Fluid Phase Equilibria 262, 23-31 (2007). [ Links ]
27. T. Loftsson, D. Hreinsdóttir, Determination of aqueous solubility by heating and equilibration: A technical note, AAPS PharmSciTech 7, 1, Article 4, E29-E34 (2006). [ Links ]
28. Y. Baena, J.A. Pinzón, H. Barbosa, F. Martínez, Temperature dependence of the solubility of some acetanilide derivatives in several organic and aqueous solvents, Phys. Chem. Liquids, 42, 603-613 (2004). [ Links ]
29. L.C. Garzón, F. Martínez, Temperature-solubility dependence for ibuprofen in some organic and aqueous solvents, J. Solution Chem., 33, 1379-1395 (2004). [ Links ]
30. C.P. Mora, F. Martínez, Solubility of naproxen in several organic solvents at different temperatures, Fluid Phase Equilibria, 255, 70-77 (2007). [ Links ]
31. J.H. Hildebrand, J.M. Prausnitz, R.L. Scott, "Regular and Related Solutions", Van Nostrand Reinhold, New York, 1970. [ Links ]
32. J.M. Hansen, "Three Dimensional solubility Parameter and Solvent Diffusion Coefficient. Importance in Surface Coating Formulation", Ph.D. Thesis, Danish Technical Press, Copenhagen, 1967. [ Links ]
33. A. Barton, "Handbook of Solubility Parameters and Other Cohesion Parameters", 2nd ed., New York, CRC Press, 1991, pp. 157-193. [ Links ]
34. C.M. Hansen, Polymer additives and solubility parameters, Progr. Org. Coat., 51, 109-112 (2004). [ Links ]
35. A. Martin, P. Bustamante, El parámetro de solubilidad en las ciencias farmacéuticas, Anal. Real Acad. Farm., 55, 175-202 (1989). [ Links ]
36. A. Martin, P. Bustamante, A.H.C. Chun, "Physical Chemical Principles in the Pharmaceutical Sciences", 4th edition, Lea & Febiger, Philadelphia, 1993. [ Links ]
37. K.A. Connors, "Thermodynamics of Pharmaceutical Systems: An Introduction for Students of Pharmacy", Wiley-Interscience, Hoboken, N.J., 2002, pp. 61-66. [ Links ]
38. A. Dallos y J. Liszi, (Liquid + liquid) equilibria of (octan-1-ol + water) at temperatures from 288.15 K to 323.15 K, J. Chem. Thermodynamics, 27, 447-448 (1995). [ Links ]
39. A. Kristl, G. Vesnaver, Thermodynamic investigation of the effect of octanol-water mutual miscibility on the partitioning and solubility of some guanine derivatives, J. Chem. Soc., Faraday Trans., 91, 995-998 (1995). [ Links ]
40. F. Martínez, A. Gómez, Thermodynamic study of the solubility of some sulfonamides in octanol, water, and the mutually saturated solvents, J. Solution Chem., 30, 909-923 (2001). [ Links ]
41. C. Tanford, "The Hydrophobic Effect: Formation of Micelles and Biological Membranes", John Wiley & Sons, New York, 1973. [ Links ]
42. Y. Baena, E.F. Vargas, F. Martínez, Thermodynamic aspects of solvation and dilution for acetanilide and phenacetin in some aqueous and organic solvents mutually saturated, Vitae U de A, 15, 132-140 (2008). [ Links ]
43. C.P. Mora, H.R. Lozano, F. Martínez, Aspectos termodinámicos de la miscibilidad parcial entre el octanol y el agua, Rev. Bras. Cienc. Farm., 41, 13-26 (2005). [ Links ]
44. A. Dinge, M. Nagarsenker, Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity, AAPS PharmSciTech, 9, 349-356 (2008). [ Links ]














