Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418On-line version ISSN 1909-6356
Rev. colomb. cienc. quim. farm. vol.39 no.2 Bogotá July/Dec. 2010
Artículo de Investigación científica
Thermodynamics of mixing of sodium naproxen and procaine hydrochloride in ethanol + water cosolvent mixtures
Estudio termodinámico del proceso de mezcla de naproxeno sódico y procaína clorhidrato en mezclas cosolventes etanol + agua
Daniel R. Delgado1, Reinaldo G. Sotomayor2, Diego R. Monterroza2, Carolina P. Mora3, Edgar F. Vargas4, Fleming Martínez5*
1 Centro de Investigaciones, Corporación Universitaria Iberoamericana, Bogotá, D. C., Colombia.
2 Laboratorios Procaps, Barranquilla, Colombia.
3 Grupo de Investigaciones Natura, Departamento de Química Farmacéutica, Facultad de Ciencias Naturales, Universidad Icesi, Santiago de Cali, Colombia.
4 Grupo de Termodinámica de Soluciones, Departamento de Química, Facultad de Ciencias, Universidad de los Andes, Bogotá, D. C., Colombia.
5 Grupo de Investigaciones Farmacéutico-Fisicoquímicas, Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia, A. A. 14490, Bogotá, D. C., Colombia.
* Corresponding Author: E-mail: fmartinezr@unal.edu.co.
Recibido para evaluación: 25 de septiembre de 2010 Aceptado para publicación: 15 de octubre de 2010
SUMMARY
Thermodynamic functions Gibbs energy, enthalpy, and entropy of mixing of sodium naproxen and procaine hydrochloride were evaluated. Mixing quantities were calculated based on fusion calorimetric values obtained from differential scanning calorimetry measurements and equilibrium solubility values reported in the literature for both drugs in ethanol + water mixtures. By means of enthalpy-entropy compensation analysis, non-linear ΔH°mix vs. ΔG°mix plots were obtained which indicates different mechanisms involved in the dissolution of these drugs according to mixtures composition. Nevertheless, the molecular and ionic events involved in the dissolution of this drug in this cosolvent system are unclear.
Key words: sodium naproxen, procaine hydrochloride, mixing process, cosolvency, ethanol, solution thermodynamics.
Resumen
En este trabajo se estudiaron las funciones termodinámicas de mezcla de naproxeno sódico y procaína clorhidrato las cuales fueron calculadas a partir de las propiedades calorimétricas de fusión y de los valores de solubilidad en equilibrio en mezclas etanol + agua publicados en la literatura. Mediante análisis de compensación entálpica- entrópica se obtienen gráficos no lineales de ΔH°mix vs. ΔG°mix indicando diferentes mecanismos en el proceso de disolución según la composición cosolvente. Sin embargo, los eventos moleculares e iónicos involucrados en el proceso de disolución de este fármaco en este sistema cosolvente no son claros.
Palabras clave: naproxeno sódico, procaína clorhidrato, proceso de mezcla, cosolvencia, etanol, termodinámica de soluciones.
Introduction
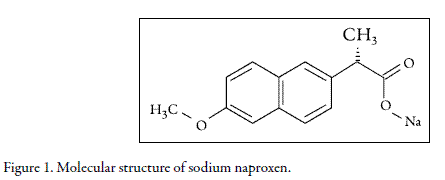
Sodium naproxen (Na-NAP, Fig. 1) is a non-steroidal anti-inflammatory drug derived of propionic acid used widely as analgesic and antipyretic although it also is used for relief of symptoms of rheumatoid arthritis and osteoarthritis in addition to treatment of dysmenorrheal, among other indications (1). Although Na-NAP is widely used nowadays in therapeutics, the physicochemical information about their aqueous solutions is not complete at present, although several physicochemical studies have been done. Thus, the solution thermodynamics in neat aqueous media for this drug (as dissociate and non-dissociate compound) has been presented in the literature (2-7). In the same way, the solution thermodynamics as non-dissociate compound in some cosolvent mixtures has also been reported (8-10). Recently, Chavez studied the solubility of Na-NAP in some methanol + water and ethanol + water mixtures at several temperatures (11); whereas, Delgado et al. developed a thermodynamic analysis of its solubility in ethanol + water mixtures (12). Moreover, the physicochemical aspects of transfer of this drug (as non-dissociate compound) from aqueous media up to octanol and some phospholipidic vesicles have also been reported (13). Besides, the apparent molar volumes in water at several concentrations and temperatures have also been reported in the literature (14).
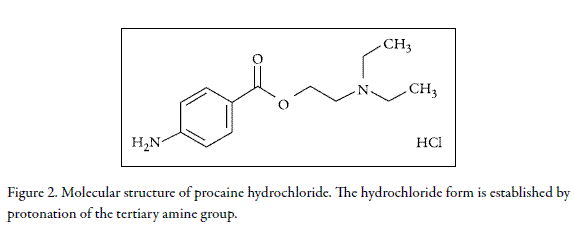
On the other hand, procaine-HCl (PC-HCl, Fig. 2) is a local anesthetic drug used in allopathic medicine (15), as well as in neural therapy (16). In the same way as Na- NAP, although PC-HCl is widely used nowadays in therapeutics, the physicochemical information about their aqueous solutions is not complete at present, although several physicochemical studies have been done. In this way, the solution thermodynamics in aqueous media for this drug has been presented in the literature (17, 18). These studies have been made by using the van't Hoff method (17) and calorimetric techniques (18). Moreover, Delgado et al. developed a thermodynamic analysis of its solubility in ethanol + water mixtures (19). On the other hand, the physical aspects of transfer of this drug from aqueous media up to phospholipidic vesicles have also been reported (20). On similar way, the surface tension in water has also been studied for this drug alone and in combination with phospholipidic monolayers (21). Ultimately, the apparent molar volumes in water have also been studied as a function of drug concentration and temperature in diluted and mean concentrated regions (22, 23), whereas the osmotic and activity coefficients of this drug in water at 298.15 K have also determined (24).
As has been already described, the solubility behavior of drugs in cosolvent mixtures is very important because cosolvent blends are frequently used in purification methods, preformulation studies, and pharmaceutical dosage forms design, among other applications (25). For these reasons, it is important to determine and collect systematically the equilibrium solubility of pharmaceutical compounds. In particular because we are still not able to predict the solubility of drugs in water, organic solvents and/or mixed solvent systems with an acceptable prediction error (26). Besides, temperature-solubility dependence allows us to carry out the respective thermodynamic analysis, which, on the other hand, also permits inside the molecular mechanisms, involved toward the solution processes (9).
In this context, the main objective of this research was to evaluate the effect of the cosolvent composition on the thermodynamics of mixing of Na-NAP and PC-HCl in some ethanol (EtOH) + water cosolvent mixtures. This study is based on both the calorimetric properties of fusion obtained by differential scanning calorimetry (DSC) and the van't Hoff treatment of equilibrium solubility values reported in the literature (12, 19).
Materials and Methods
Reagents
Sodium naproxen (d-2-(6-methoxy-2-naphthyl) propionic acid sodium salt; CAS: [26159-34-2]; purity: 0.9990 in mass fraction) and Procaine-HCl (4-Aminobenzoic acid 2-(diethylamino)ethyl ester HCl; CAS: [51-05-8]; purity: 0.9995 in mass fraction) (27) used were in agreement with the quality requirements indicated in the American Pharmacopeia, USP (28).
Calorimetric study
Melting point and enthalpy of fusion of both drugs were determined by DSC studies (DSC 823E Mettler Toledo). Thermal analyses were performed at a heating rate of 10K min -1 in a dynamic nitrogen atmosphere (60 mL min -1). Nearly 1.5 mg of Na-NAP or PC-HCl was used. The equipment was calibrated using Indium as standard (29).
Results and Discussion
Before to show the results, it is important to consider that both drugs have electrolyte behavior (12, 19), and thus, they dissociate in aqueous solution interacting with the cosolvent mixture by strong ion-dipole interactions, as well as by other weak non covalent interactions because of their nonpolar groups. On this way, Na-NAP could acts as a Lewis base (-OCH3, Fig. 1), in order to establish hydrogen bonds with protondonor functional groups in the solvents (-OH groups); whereas PC-HCl could acts as a Lewis acid (-NH2 group) or Lewis base (-NH2 and -COO- groups), in order to establish hydrogen bonds with proton-acceptor or donor functional groups in the solvents (-OH groups) as well (30, 31).
Ideal solubility of both drugs
The ideal solubility of non-electrolyte crystalline solutes in a liquid solvent can be calculated by means of Equation 1:
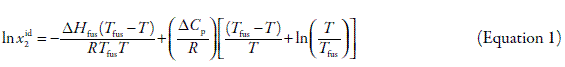
where xid2 is the ideal solubility of the solute as mole fraction, ΔHfus is the molar enthalpy of fusion of the pure solute (at the melting point), Tfus is the absolute melting point, T is the absolute solution temperature, R is the gas constant (8.314 J mol-1 K-1), and ΔCp is the difference between the molar heat capacity of the crystalline form and the molar heat capacity of the hypothetical supercooled liquid form, both at the solution temperature (32). Since ΔCp cannot be easy experimentally determined it is usual assuming that it may be approximated to the entropy of fusion, calculated as ΔSfus = ΔHfus/Tfus. The main reasons for the last assumption have been well discussed in the literature (33). Although Equation 1 was developed for non electrolyte compounds, it has also been used to estimate ideal solubilities of some electrolyte drugs (34, 35).
In this context, Tables 1 and 2 summarize the thermodynamic properties of fusion and the ideal solubilities of both drugs. Table 2 shows that ideal solubilities of Na-NAP are lower than those reported for molecular NAP (6); as an example at 298.15 K these values are 4.098 x 10-2 for molecular NAP in front to 8.88 x10-3 for Na-NAP. In different way, no values of ideal solubility have been reported for procaine base.

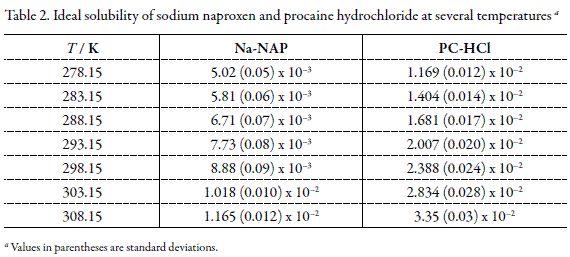
On the other hand, the experimental equilibrium solubility values for both drugs have been reported in the literature (12, 19).
Thermodynamic quantities of solution of both drugs
Because the drugs considered in this research are electrolyte compounds, it is important to keep in mind that in general terms, it could be stated that a strong electrolyte dissociates according to the expression, Cv+Avâ→v+Cz++v_Azâ, where v+ is the number of cations (Cz+) of valence z+ and v_ is the number of anions (Az-) of valence z-. Because is not possible to determine experimentally the activity of ions separately, the concept of mean ionic activity (av±) is used. Thus, the thermodynamic activity for an electrolyte can be defined as, a2=av++av--=av± (36-38).
Both drugs studied here are electrolyte solutes of type one-one, that is, they dissociate in aqueous solutions to generate two species, a monovalent anion and a monovalent cation, respectively. If the inter-ionic interactions are not considered, in a first approach the v value could be ideally assumed as 2 for these drugs, and thus this value could be used to calculate the apparent thermodynamic functions of solution (12, 19).
In this way, according to van't Hoff analysis, the apparent standard enthalpy change of solution (ΔH0soln) for electrolytes type one-one, if the inter-ionic interactions are not considered, is obtained by using the mean harmonic temperature (Thm is 292.8 K in our case) according to Equation 2 (12, 19).

where, R is the universal gas constant (8.314 J mol-1K-1). In all cases studied, linear models with good determination coefficients were obtained.
The apparent standard Gibbs energy change for the solution process (ΔG0soln) of electrolytes type one-one, considering the approach proposed by Krug et al. (39), is calculated at mean harmonic temperature by means of,

in which, the intercept used is the one obtained in the analysis by treatment of ln XDrug as a function of 1/T-1/Thm. Finally, the apparent standard entropic change for solution process (ΔS0soln) is obtained from the respective ΔH0soln and ΔG0soln values by using:

Table 3 summarizes the apparent standard thermodynamic functions for experimental solution process both drugs in all EtOH + water cosolvent mixtures. In order to calculate the thermodynamic quantities for the experimental solution processes some propagation of uncertainties' methods were used (40). It is found that standard Gibbs energies, apparent enthalpies, and entropies of solution for both drugs are positive in all cases, and therefore the dissolution processes are always endothermic and driven by the entropy of solution.
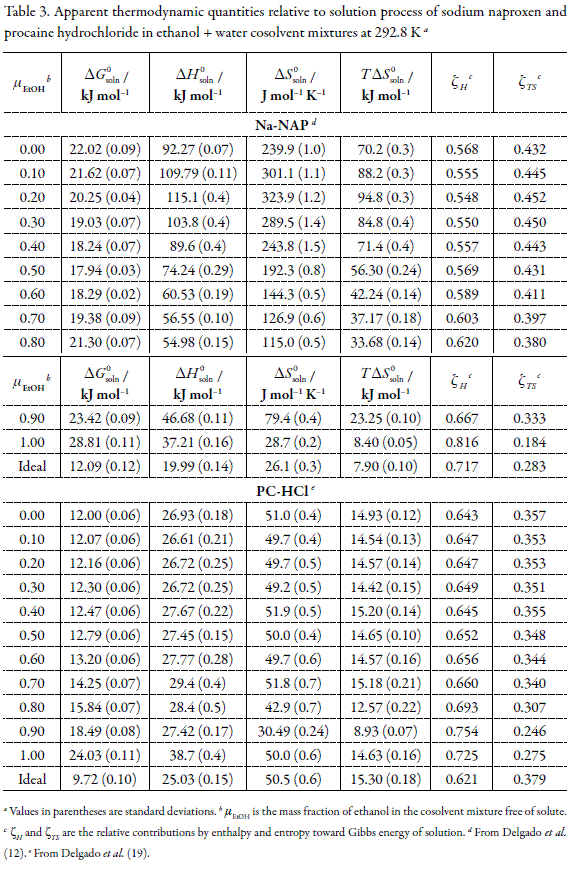
With the aim to compare the relative contributions by enthalpy (ζH) and by entropy (ζTS) toward the solution process, equations 5 and 6 were employed, respectively (3).


From Table 3 it follows that the main contributor to standard Gibbs energy of solution process of both drugs is the enthalpy in particular in EtOH- rich mixtures indicating the relevance of the energetic factor on the dissolution processes of these drugs in this cosolvent system.
Thermodynamic quantities of mixing of both drugs
The solution process may be represented by the following hypothetical stages (9):
Solute(Solid)→ Solute(Liquid)→Solute(Solution)
where, the respective partial processes toward the solution are solute fusion and mixing at the same temperature (292.8 K), which permits to calculate the partial thermodynamic contributions to overall solution process by means of equations 7 and 8, respectively.


where, ΔH292.8fus and ΔS292.8fus represent the thermodynamic functions of fusion process at harmonic temperature (292.8 K). The ΔH292.8fus values were calculated from ΔHTfus= ΔHMPfus-ΔCp/Tfus-T), by using ΔSMPfus instead of ΔCp, obtaining the values of 20.01 and 25.04 kJ mol-1 for Na-NAP and PC-HCl, respectively, which are coincident with the enthalpic changes for ideal solution processes (Table 3). In contrast, the entropies of fusion at 292.8 K (68.3 and 85.5 J mol-1 K-1 for Na-NAP and PC-HCl, respectively) are not coincident with the entropic changes of the ideal solution processes at this temperature (Table 3). For this reason, for practical purposes in this analysis, the ΔS0idsoln values were used instead of ΔS292.8soln as was made previously with several non electrolyte drugs in EtOH + water cosolvent mixtures (9, 41-43). Table 4 summarizes the thermodynamic quantities of mixing for both drugs. Gibbs energy of mixing is positive in all the systems studied apparently indicating non spontaneity on liquids mixing.
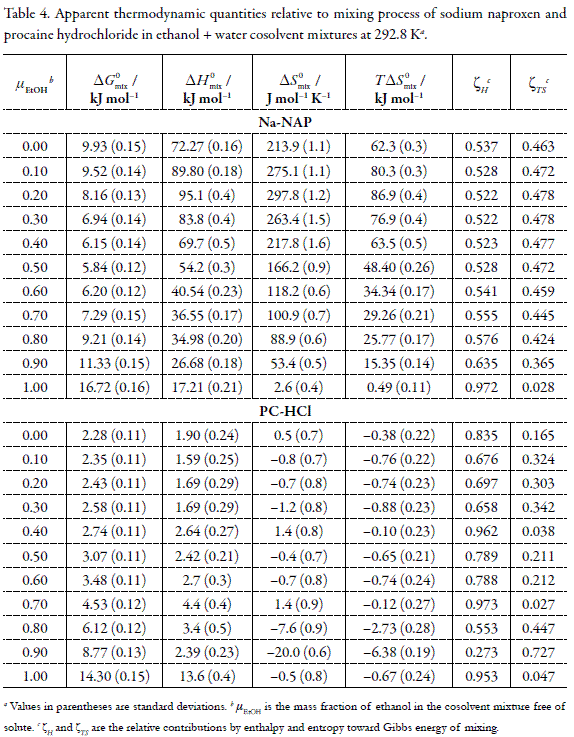
By analyzing the partial contributions by ideal solution (related to solute fusion process) and mixing processes to the enthalpy and entropy of solution, it is found that ΔH292.8fus and ΔS292.8fus are positive (Table 4), while the contribution of the thermodynamic functions relative to mixing process toward the solution process is variable. In this context, ΔH0mix is positive for both drugs in all mixtures being strongly endothermic for Na-NAP and slightly endothermic for PC-HCl; while the entropy of mixing (ΔS0mix) is positive in all mixtures for Na-NAP and negative for PC-HCl in all systems except in water and the mixture of 0.70 in mass fraction of EtOH. It is important to note that the mixing process of PC-HCl is almost isentropic in the interval 0.00 ≤µEtOH≤ 0.70 and neat EtOH. By considering the contribution of mixing processes toward the dissolution processes, it could be concluded that, i) for Na-NAP the mixing process is driven by entropy (ΔH0mix>0 and ΔS0mix>0) and ii) for PC-HCl nor enthalpy or entropy driven is found ((ΔH0mix>0 and ΔS0mix<), except in neat water and the mixtures of 0.40 and 0.70 in mass fraction of EtOH where entropy-driving is found because of the positive entropies of mixing.
The net variation in ΔH0mix values results from the contribution of several kinds of interaction. The enthalpy of cavity formation (required for solute accommodation) is endothermic because energy must be supplied against the cohesive forces of the solvent. This process decreases solubility. On the other hand, the enthalpy of solutesolvent interaction is exothermic and results mainly from ion-dipole, van der Waals and Lewis acid-base interactions. In the case of non-electrolyte drugs, the structuring of water molecules around the nonpolar groups of solutes (hydrophobic hydration) contributes to lower the net heat of mixing to small or even negative values in aqueous solutions as it is the case of PC-HCl (Table 4). Nevertheless, these drugs are electrolyte and therefore interact with the solvent by ion-dipole interactions which could lead to hydrophilic hydration around the anionic or cationic groups.
As it was already said, the energy of cavity formation should be lower as the proportion of EtOH increases because the polarity of the medium decreases, a fact that favors solute-solvent interactions except ion-dipole. This fact is observed in Table 4, where ΔH0mix for Na-NAP is lower as the proportion of cosolvent increases from 0.20 in mass fraction of EtOH. In different way, the behavior of PC-HCl is more complicate. According to Romero et al. (44) in the initial portion of the solubility curve, the hydrogen bonding of the drug will increase with EtOH concentration. At large cosolvent proportions, this interaction may be saturated, becoming a constant contribution. On the other hand, nonspecific and cavity effects are not saturated and vary with EtOH concentration. Nevertheless, these considerations do not explain the behavior observed in water-rich mixtures where the ion-dipole interactions predominate for both electrolyte drugs.
Enthalpy-Entropy compensation of mixing of both drugs
According to the literature, the making of weighted graphs of ΔH0-appsoln as a function of ΔG0-appsoln at mean harmonic temperature allows us to observe similar mechanisms for the solution process according to the tendencies obtained (45, 46).
In this context, Figure 3 shows fully that Na-NAP and PC-HCl in the EtOH + water cosolvent system present non-linear ΔH0mix vs. ΔG0mix compensation with negative slope in the intervals 0.00≤µEtOH≤0.20 and 0.50≤µEtOH≤1.00 and positive slope in the interval 0.20≤µEtOH≤0.50 for Na-NAP. Accordingly to this graph it follows that the driving function for the mixing processes of this drug is the entropy in the former cases, while in the second case, the driving function is the enthalpy. Nevertheless, the molecular and ionic events involved in the dissolution of this anionic drug in this cosolvent system are unclear. On the other hand, the behavior obtained for PC-HCl is more complex compared with Na-NAP, in particular in the water-rich region, where non clear tendencies are observed, thereby, the ionic and molecular events involved are also unclear for this cationic drug.
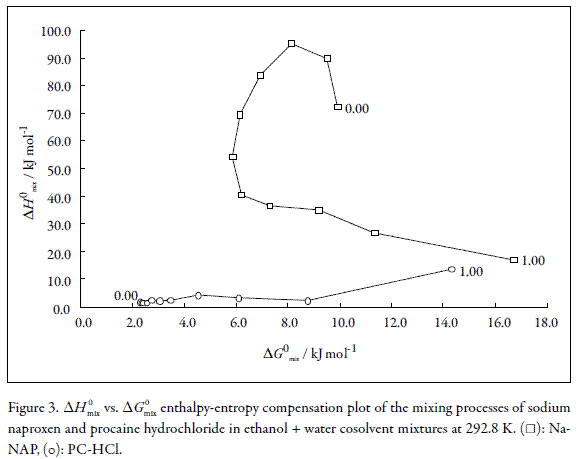
Conclusions
From all topics discussed previously it can be concluded that the mixing process of Na-NAP and PC-HCl in EtOH + water mixtures is variable depending on the cosolvent composition. Non linear enthalpy-entropy compensation was found for these drugs in this cosolvent system. Despite of the thermodynamic treatment made the ionic and molecular events involved in the dissolution processes of these drugs are unclear. Ultimately, it can be said that the data presented in this report expand the physicochemical information about electrolyte drugs in aqueous and alcoholic solutions.
Acknowledgments
We thank the DIB of the Universidad Nacional de Colombia (UNC) for the financial support. Additionally we thank the Department of Pharmacy of UNC and the Department of Chemistry of the Universidad de los Andes for facilitating the equipment and laboratories used.
References
1. R.B. Raffa, Analgesic, antipyretic, and anti-inflammatory drugs, in "Remington: The Science and Practice of Pharmacy", 21st edition, edited by A.R. Gennaro, Lippincott Williams & Wilkins, Philadelphia, 2005. [ Links ]
2. P.D. Martino, C. Barthelemy, G.F. Palmieri, S. Martelli, Physical characterization of naproxen sodium hydrate and anhydrate forms, Eur. J. Pharm. Sci., 14, 293- 300 (2001). [ Links ]
3. G.L. Perlovich, S.V. Kurkov, A.N. Kinchin, A. Bauer-Brandl, Thermodynamics of solutions III: Comparison of the solvation of (+)-naproxen with other NSAIDs, Eur. J. Pharm. Biopharm., 57, 411-420 (2004). [ Links ]
4. J.R. Méndez del Río, "Solubility and phase transitions in batch and laminar-flow tubular crystallizers", M.Sc. thesis, School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, Atlanta, 2004. [ Links ]
5. Y.S. Kim, J.R. Méndez del Río, R.W. Rousseau, Solubility and prediction of the heat of solution of sodium naproxen in aqueous solutions, J. Pharm. Sci., 94, 1941-1948 (2005). [ Links ]
6. C.P. Mora, F. Martínez, Thermodynamic quantities relative 6. to solution processes of naproxen in aqueous media at pH 1.2 and 7.4, Phys. Chem. Liq., 44, 585-596 (2006). [ Links ]
7. C.P. Mora, H.J. Barbosa, F. Martínez, Efecto de algunos solventes orgánicos en saturación sobre las funciones termodinámicas de disolución del naproxén en medios acuosos a pH fisiológico, Vitae, Rev. Fac. Quím. Farm., 14, 38-47 (2007). [ Links ]
8. P. Bustamante, M.A. Peña, J. Barra, Partial solubility parameters of naproxen and sodium diclofenac, J. Pharm. Pharmacol., 50, 975-982 (1998). [ Links ]
9. D.P. Pacheco, F. Martínez, Thermodynamic analysis of the solubility of naproxen in ethanol + water cosolvent mixtures, Phys. Chem. Liq., 45, 581-595 (2007). [ Links ]
10. Y.J. Manrique, D.P. Pacheco, F. Martínez, Thermodynamics of mixing and solvation of ibuprofen and naproxen in propylene glycol + water cosolvent mixtures, J. Solution Chem., 37, 165-181 (2008). [ Links ]
11. K.J. Chavez, "Crystallization of pseudopolymorphic forms of sodium naproxen in mixed solvent systems", Ph.D. thesis, School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, Atlanta, 2009. [ Links ]
12. D.R. Delgado, M.A. Ruidiaz, S.M. Gómez, M. Gantiva, F. Martínez, Thermodynamic study of the solubility of sodium naproxen in some ethanol + water mixtures, Quím. Nova, 33, 1923-1927 (2010). [ Links ]
13. C.P. Mora, F. Martínez, Thermodynamic study of partitioning and solvation of (+)-naproxen in some organic solvent/buffer and liposome systems, J. Chem. Eng. Data, 52, 1933-1940 (2007). [ Links ]
14. A.R. Holguín, D.R. Delgado, M.A. Ruidiaz, E.F. Vargas, F. Martínez, Apparent molar volumes of sodium naproxen in water at several concentrations and temperatures, Lat. Am. J. Pharm., aceptado para publicación. [ Links ]
15. M.R. Borenstein, Local anesthetics, in "Remington: The Science and Practice of Pharmacy", 21st edition, edited by A.R. Gennaro, Lippincott Williams & Wilkins, Philadelphia, 2005. [ Links ]
16. M.P. Dosch, "Atlas of Neural Therapy with Local Anesthetics", 2nd edition, Georg Thieme Verlag, Sttutgart, 2003. [ Links ]
17. I. Labastidas, F. Martínez, Aspectos termodinámicos de la solubilidad acuosa de algunas sales orgánicas de interés farmacéutico, Acta Farm. Bonaerense, 25, 55-63 (2006). [ Links ]
18. D.R. Torres, L.H. Blanco, E.F. Vargas, F. Martínez, Calorimetric enthalpies of solution for lidocaine-HCl and procaine-HCl in water at 298.15 K, J. Chem. Eng. Data, 54, 786-790 (2009). [ Links ]
19. D.R. Delgado, E.F. Vargas, F. Martínez, Thermodynamic study of the solubility of procaine-HCl in some ethanol + water cosolvent mixtures, J. Chem. Eng. Data, 55, 2900-2904 (2010). [ Links ]
20. A. Avdeef, K.J. Box, J.E.A. Comer, C. Hibbert, K.Y. Tam, pH-Metric logP 10. Determination of liposomal membrane-water partition coefficients of ionizable drugs, Pharm. Res., 15, 209-215 (1998). [ Links ]
21. F.A. Vilallonga, E.W. Phillips, Surface activities of procaine, lidocaine, and tetracaine and their interaction energies with phospholipid monolayers, J. Pharm. Sci., 68, 314-316 (2006). [ Links ]
22. D.R. Torres, L.H. Blanco, F. Martínez, E.F. Vargas, Apparent molal volumes of lidocaine-HCl and procaine-HCl in aqueous solution as a function of temperature, J. Chem. Eng. Data, 52, 1700-1703 (2007). [ Links ]
23. D.R. Delgado, A.F. Jiménez-Kairuz, R. Manzo, E.F. Vargas, F. Martínez, Apparent molar volumes of the anesthetics procaine-HCl and lidocaine-HCl in water at temperatures from 278.15 to 313.15 K, Rev. Colomb. Cienc. Quím. Farm., 39, 57-67 (2010). [ Links ]
24. D.R. Torres, L.H. Blanco, E.F. Vargas, F. Martínez, Osmotic and activity coefficients of aqueous solutions of the anesthetic drugs lidocaine-HCl and procaine- HCl at 298.15 K, Lat. Am. J. Pharm., 29, 1250-1253 (2010). [ Links ]
25. J.T. Rubino, Cosolvents and Cosolvency, in "Encyclopedia of Pharmaceutical Technology", Vol. 3, edited by J. Swarbrick, J.C. Boylan, Marcel Dekker, Inc., New York, 1988. pp. 375-398. [ Links ]
26. A. Jouyban, Solubility: still a challenging subject in pharmaceutical sciences, Vitae, Rev. Fac. Quím. Farm., 17, 241-242 (2010). [ Links ]
27. S. Budavari, M.J. O'Neil, A. Smith, P.E. Heckelman, J.R. Obenchain Jr., J.A.R. Gallipeau, M.A. D'Arecea, "The Merck Index, An Encyclopedia of Chemicals,Drugs, and Biologicals", 13th edition, Merck & Co., Inc., Whitehouse Station, NJ, 2001. [ Links ]
28. US Pharmacopeia, 23rd edition, United States Pharmacopeial Convention, Rockville, MD, 1994. [ Links ]
29. S.A. McCauley, H.G. Brittain, Thermal methods of analysis, in "Physical Characterization of Pharmaceutical Solids", edited by H.G. Brittain, Marcel Dekker, Inc., New York, 1995. [ Links ]
30. A. Martin, P. Bustamante, A.H.C. Chun, "Physical Chemical Principles in the Pharmaceutical Sciences", 4th edition, Lea & Febiger, Philadelphia, 1993. [ Links ]
31. A.T. Florence, D. Atwood, "Physicochemical Principles of Pharmacy", 3rd edition, MacMillan Press Ltd., London, 1998. pp 64-67. [ Links ]
32. J.H. Hildebrand, J.M. Prausnitz, R.L. Scott, "Regular and Related Solutions", Van Nostrand Reinhold, New York, 1970. [ Links ]
33. S.H. Neau, G.L. Flynn, Solid and liquid heat capacities of n-alkyl para-aminobenzoates near the melting point, Pharm. Res., 7, 1157-1162 (1990). [ Links ]
34. P. Bustamante, M.A. Peña, J. Barra, The modified extended Hansen method to determine partial solubility parameters of drugs containing a single hydrogen bonding group and their sodium derivatives: benzoic acid/Na and ibuprofen/ Na, Int. J. Pharm., 194, 117-124 (2000). [ Links ]
35. P. Guerrieri, A.C.F. Rumondor, T. Li, L.S. Taylor, Analysis of relationships between solid-state properties, counterion and developability of pharmaceutical salts, AAPS PharmSciTech, 11, 1212-1222 (2010). [ Links ]
36. I.M. Klotz, R.M. Rosenberg, "Chemical Thermodynamics: Basic Theory and Methods", 6th edition, John Wiley & Sons, Inc., New York, 2000. pp 438-448. [ Links ]
37. J. Bevan, J. Boerio-Goates, "Chemical Thermodynamics: Principles and Applications", Academic Press, New York, 2000. pp 295-301. [ Links ]
38. K.A. Connors, "Thermodynamics of Pharmaceutical Systems: An Introduction for Students of Pharmacy", Wiley-Interscience, Hoboken NJ, 2002. pp 96-105. [ Links ]
39. R.R. Krug, W.G. Hunter, R.A. Grieger, Enthalpy-entropy compensation. 2. Separation of the chemical from the statistical effects, J. Phys. Chem., 80, 2341- 2351 (1976). [ Links ]
40. P.R. Bevington, "Data Reduction and Error 40. Analysis for the Physical Sciences", McGraw-Hill Book Co., New York, 1969. pp 56-91. [ Links ]
41. J.A. Jiménez, F. Martínez, Thermodynamic magnitudes of mixing and solvation of acetaminophen in ethanol + water cosolvent mixtures, Rev. Acad. Colomb. Cienc., 30, 87-99 (2006). [ Links ]
42. J. Manrique, F. Martínez, Solubility of ibuprofen in some ethanol + water cosolvent mixtures at several temperatures, Lat. Am. J. Pharm., 26, 344-354 (2007). [ Links ]
43. M. Gantiva, A. Yurquina, F. Martínez, Solution thermodynamics of ketoprofen in ethanol + water cosolvent mixtures, J. Chem. Eng. Data, 55, 113-118 (2010). [ Links ]
44. S. Romero, A. Reillo, B. Escalera, P. Bustamante, The behavior of paracetamol in mixtures of amphiprotic and amphiprotic-aprotic solvents: Relationship of solubility curves to specific and nonspecific interactions, Chem. Pharm. Bull., 44, 1061-1064 (1996). [ Links ]
45. P. Bustamante, S. Romero, A. Peña, B. Escalera, A. Reillo, Nonlinear enthalpy-entropy compensation for the solubility of drugs in solvent mixtures: paracetamol, acetanilide and nalidixic acid in dioxane-water, J. Pharm. Sci., 87, 1590-1596 (1998). [ Links ]
46. E. Tomlinson, Enthalpy-entropy compensation analysis of pharmaceutical, biochemical and biological systems, Int. J. Pharm., 13, 115-144 (1983). [ Links ]














