Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418On-line version ISSN 1909-6356
Rev. colomb. cienc. quim. farm. vol.39 no.2 Bogotá July/Dec. 2010
Artículo de Investigación
Correlating the solubility of indomethacin in 1,4-dioxane + water mixtures by means of the Jouyban-Acree model
Correlación de la solubilidad de indometacina en mezclas 1,4-dioxano + agua mediante el modelo de Jouyban-Acree
Miller A. Ruidiaz 1 , Daniel R. Delgado 2 , Fleming Martínez 3
1 Departamento de Ciencias Básicas, Corporación Tecnológica de Bogotá, Bogotá, D. C., Colombia.
2 Centro de Investigaciones, Corporación Universitaria Iberoamericana, Bogotá, D. C., Colombia.
3 Grupo de Investigaciones Farmacéutico-Fisicoquímicas, Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia, A. A. 14490, Bogotá, D. C., Colombia. E-mail: fmartinezr@unal.edu.co.
* E-mail: fmartinezr@unal.edu.co.
Recibido para evaluación: 13 de octubre de 2010 Aceptado para publicación: 15 de noviembre de 2010
SUMMARY
In this work the validity of the Jouyban-Acree and Yalkowsky-Roseman models is evaluated to correlate the solubility of indomethacin in 1,4-dioxane + water cosol- vent mixtures. The solubility correlation is studied as a function of temperature and cosolvent composition. Both models require only the experimental solubility values in the pure solvents at all the temperatures evaluated. The solubility calculated values by using both models deviate notoriously from experimental values in several cases.
Key words: indomethacin, 1,4-dioxane + water mixtures, Jouyban-Acree and Yalkowsky-Roseman models.
RESUMEN
En este trabajo se evaluó la utilidad de los modelos Jouyban-Acree y Yalkowsky- Roseman en la correlación de la solubilidad de la indometacina en mezclas cosolventes 1,4-dioxano + agua. La correlación de la solubilidad se estudió en función de la temperatura y la composición cosolvente. Los dos modelos requieren únicamente los valores de solubilidad en los solventes puros a todas las temperaturas de interés. Los valores calculados se desvían significativamente de los experimentales en muchos casos.
Palabras clave: indometacina, mezclas 1,4-dioxano + agua, modelos de Jouyban- Acree y Yalkowsky-Roseman.
INTRODUCTION
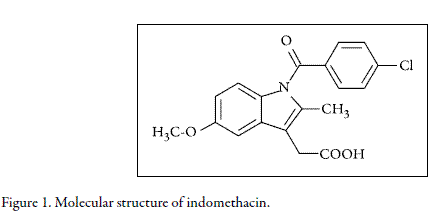
Indomethacin (IMC, Fig. 1) is an anti-inflammatory drug sometimes used in actual therapeutics whose physicochemical properties have not been thoroughly studied (1, 2). In this context, it is well known that several physicochemical properties such as, the solubility and occupied volumes by active ingredients and excipients are important for all the pharmaceutical scientists, because they facilitate the processes associated to design and development of new products (3). Moreover, the reported techniques intended to predict these values are highly appreciated for practical applications because they diminish the economic and experimental efforts. Thes e consideration s imply significant reductions in costs during the design and development stages at industrial level (4).
For these reasons, the main objective of this study was to evaluate the usefulness of Jouyban-Acree model (5) to correlate the equilibrium solubility of IMC in binary mixtures conformed by 1,4-dioxane and water as a function of the solvent composition and temperature. In similar way, the log-linear model proposed by Yalkowsky and Roseman (6) was also challenged in front to the experimental solubility values of this drug.
It is known that 1,2-propanediol and ethanol are the cosolvents most widely used in drug formulation design, especially those intended for peroral and parenteral administration (7, 8). Several examples of pharmaceutical formulations using these cosolvents have been presented by Rubino (7). 1,2-propanediol and ethanol are hydrogen-donor and hydrogen-acceptor solvents, and have relatively large dielectric constants, 24 and 32 at 293.15 K, respectively (9). Therefore, mixtures with low polarities could not be studied by using these two solvents when blended with water.
On the other hand, 1,4-dioxane is miscible with water in all possible compositions, although it has a low dielectric constant, 2.2 at 293.15 K (9). When this solvent is blended with water allows studying dielectric constants from 2 to 80 at room temperature. 1,4-dioxane acts just as a Lewis base in aqueous media in different way to 1,2-propanediol and ethanol that act as both Lewis donors and acceptors. Although it is a toxic solvent, it has been widely used as a model cosolvent for solubility studies of drugs by several authors (10-12).
THEORETICAL
The different strategies intended to estimate physicochemical properties of drugs are highly valued at industrial level. Several methods to estimate the solubility in solvent mixtures have been reported in the pharmaceutical and chemical literature (13, 14). Some of them have been challenged recently in the correlation of the equilibrium solubility of several drugs (4, 15).
The simplest model to predict drug solubility in cosolvent mixtures is the one based on the algebraic rule of mixing, which for semipolar compounds in binary mixtures takes the following form:
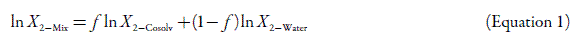
where X 2-Mix is the drug solubility calculated in the cosolvent mixture considered, X 2-Cosolv is the drug solubility in the neat cosolvent, X 2-Water is the drug solubility in neat water, and f is the volume fraction of cosolvent in the mixture free of drug dissolved. This last term is calculated assuming additive volumes according to:

where, V Cosolv and V Water are the respective volumes of cosolvent and water (16). Equation 1 is a practical form of the logarithmic-linear model developed by Yalkowsky and Roseman (6), which has the form:

where S 2-Mix and S 2-Water are the solubilities (as molarity or mole fraction) in the cosolvent mixture and water, respectively, and σ is the solubilizing power factor in the same solute-solvent system. The σ term in equation 3 has been correlated with several polarity indexes such as, octanol-water partition coefficients, Hildebrand solubility parameters, and interfacial tensions, among others (17).
Nevertheless, it was found experimentally that the behavior of several lipophilic solutes deviate notoriously from this simple additive rule of solubility, in particular when the solvents used are amphiprotic. As an example, in the case of propylene glycol + water mixtures, Rubino and Obeng (18) found negative deviations to equation 1 in water-rich mixtures and positive deviations in propylene glycol-rich mixtures by studying the solubility of homologous series of some alkyl p-hydroxibenzoates and p-aminoben-zoates. These authors suggested that the observed deviations were due to cosolvent- water interactions, and thereby, they exposed that cosolvent interact with water by two mechanisms, namely, (i) hydrophobic hydration by forming water "icebergs" around the non-polar groups in the cosolvent, and (ii) specific interaction between the cosolvent hydroxyl group and water molecules by hydrogen bonding, which could increase the water-structure formation obtained because of the hydrophobic effect. Thus, both interactions lead to diminish the solute-solvent interactions, and therefore, the drug solubility. Opposite, in those mixtures with high cosolvent proportion the hydrogen bonding among cosolvent and water is also present but the water-structure formation has diminished or it has disappeared.
As good attempt to consider the deviations non taken into account by Equation 1 Jouyban and Acree proposed equation 4, where T is the absolute temperature and J i are the respective polynomial coefficients. J i coefficients have theoretical meaning because each one of them is a function of the interaction energies among two and three bodies, which in turn describe the attractions among the different molecules present in solution. Equation 4 is derivate from the equation originally proposed by Redlich and Kister (19), and its development as well as its meaning has been described previously in the literature (20, 21).
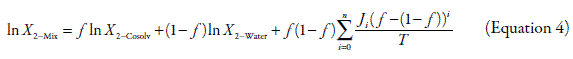
Recently, Jouyban and Acree (5) processed by regression analysis the reported solubility values (as mole fraction) of several drugs in 1,4-dioxane + water mixtures in front to equation 4, obtaining the equation 5, whose coefficients were statistically significant with p < 0.05 according to the Student's t-test.
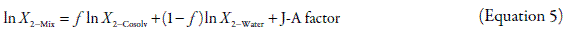
where the Jouyban-Acree factor is defined according to
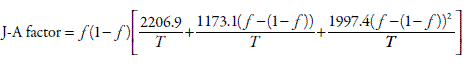
The mean percentage deviation obtained by applying Equation 5 to 36 solubility data sets was 27% which is considered as good for practical purposes.
EXPERIMENTAL
Reagents and Materials
In this investigation the following reagents and materials were used: indomethacin accomplishing the British Pharmacopoeia quality requirements (22), 1,4-dioxane A.R. Scharlau, distilled water with conductivity < 2 µS cm -1 , molecular sieve Merck (numbers 3 and 4, pore size 0.3 and 0.4 nm, respectively), and Durapore® 0.45 µm filters from Millipore Corp.
Solvent mixtures preparation
All 1,4-dioxane + water solvent mixtures were prepared by mass, using an Ohaus Pioneer TM PA214 analytical balance with sensitivity ± 0.1 mg, in quantities of 50 g. The mass fractions of 1,4-dioxane of the twelve binary mixtures prepared varied by 0.10 from 0.10 to 0.70 and by 0.05 from 0.75 to 0.95.
Solubility determination
An excess of IMC was added to approximately 10 g of each solvent mixture or neat solvent, in stoppered dark glass flasks. Solid-liquid mixtures were placed with stirring in a thermostatic mechanical shaker ( Julabo SW23) kept at 303.15, 308.15, or 313.15 (± 0.05) K or placed in re-circulating thermostatic baths (Neslab RTE 10 Digital One Thermo Electron Company) kept at 293.15 or 298.15 (± 0.05) K for at least 7 days to reach the equilibrium. In the case of neat water or water-rich mixtures the equilibration time was 14 days. These equilibrium times were established by measuring the drug concentrations till they became constant. After this time the supernatant solutions were filtered (at isothermal conditions) to ensure that they were free of particulate matter before sampling. Drug concentrations were determined after appropriate dilution by measuring the light absorbance and interpolation from a previously constructed UV spectrophotometry calibration curve (UV/VIS BioMate 3 Thermo Electron Company spectrophotometer). All the solubility experiments were run in triplicate at least.
Deviation calculations
As a deviation criterion between single experimental and calculated values by means of the Yalkowsky-Roseman and Jouyban-Acree models (5), the individual percentage deviations (IPD) were calculated according to:

On similar way, as a general criterion of the usefulness of both equations the mean percentage deviations (MPD) were calculated by means of the equation 7, where n is the number of mixtures compositions considered.

RESULT AND DISCUSSION
It is well known that the volume expressions of mixtures concentration are dependent on temperature because the volumes of liquids change with temperature according to their thermal volume expansion coefficient s (α). For this reason, Table 1 shows the temperature dependence of volume fraction in 1,4-dioxane + water mixtures with the mass composition varying in 0.10 from 0.10 to 0.70 and by 0.05 from 0.75 to 0.95 in mass fraction (µDioxane). The respective statistical description is also showed. Although the αvalues for 1,4-dioxane and water are different, 1.062 x 10 -3 K -1 and 2.51 x 10 -4 K -1 , respectively (23), the temperature dependence of f with temperature is relatively low, being in the nine cases lower than 0.30 %, which for practical purposes is considered as almost insignificant. In all cases this variation is lower than 0.60 % and the mean values obtained at temperatures from 293.15 to 313.15 K are concordant with those reported at 303.15 K. For this reason the volume fractions obtained at 303.15 K were used in all calculations as has been made in other similar studies (24-27).
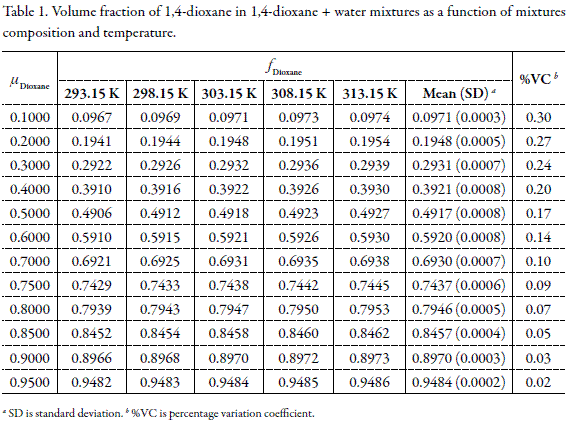
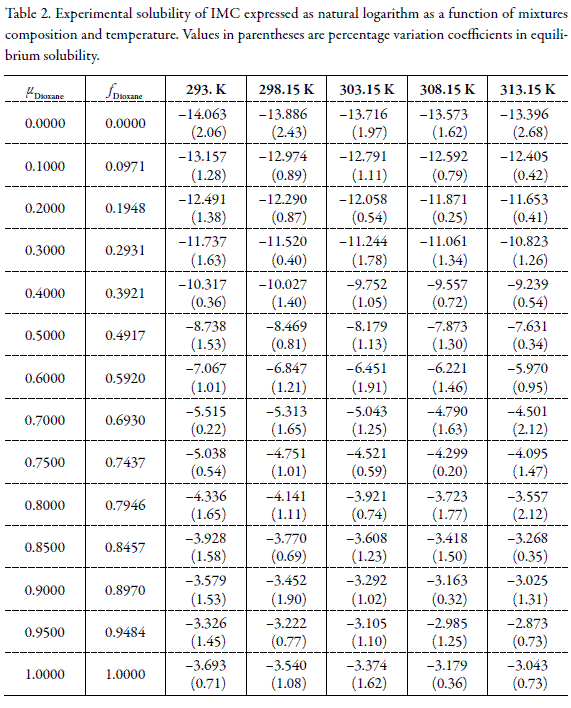
Table 2 shows the experimental values of equilibrium solubility for this pharmaceutical compound expressed as decimal logarithms of mole fraction. The values used as input in equations 1 and 5 were those obtained in the neat solvents at all temperatures studied.
Tables 3 and 4 show the values of logarithmic solubility calculated by means of equations 1 and 5 as a function of the mixtures composition and temperature, respectively. Individual and group percentage deviations with respect to equilibrium solubilities are also showed in these tables.
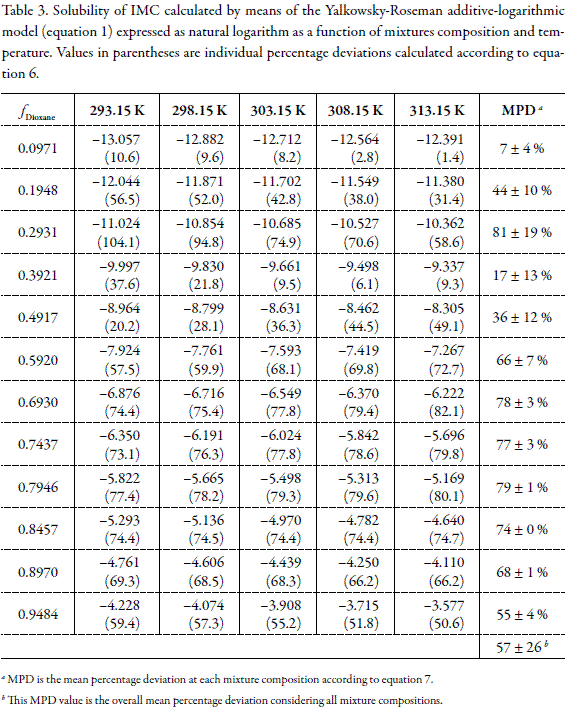
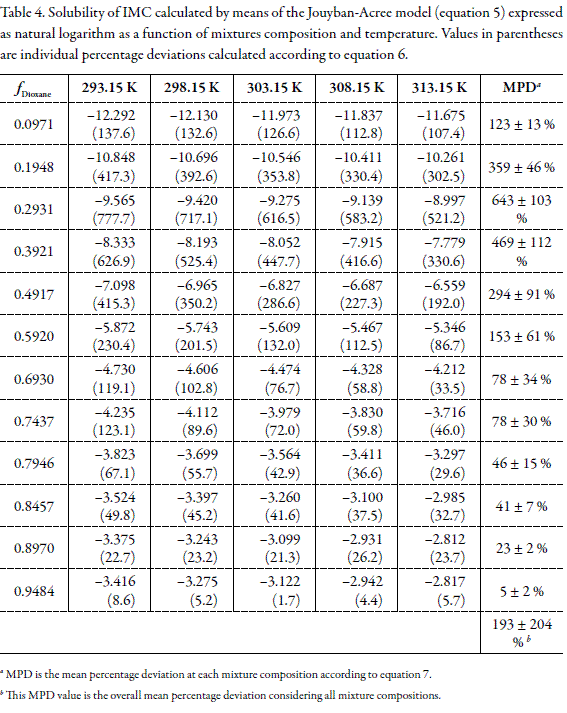
By comparing the predictive results obtained for this drug by using both models it is clear that Jouban-Acree model (equation 5) is not better than additive behavior (equation 1), because of their MPD values, namely, 193 ± 204 % in the first case, in front to 57 ± 26 % in the case of equation 1. Thus, neither Yalkowsky-Roseman nor Jouyban-Acree models would be useful at industrial level if equilibrium solubility estimations within 30 or 40 % in uncertainty are allowed in the research and development of homogeneous liquid products in the pharmaceutical industry.
To see more clearly these effects, Figure 2 shows the difference s obtaine d between experimental solubilities for IMC at 298.15 K in front to those calculated by means of equation 1. In similar way, Figure 2 also shows the differences obtained between equations 1 and 5, respectively.
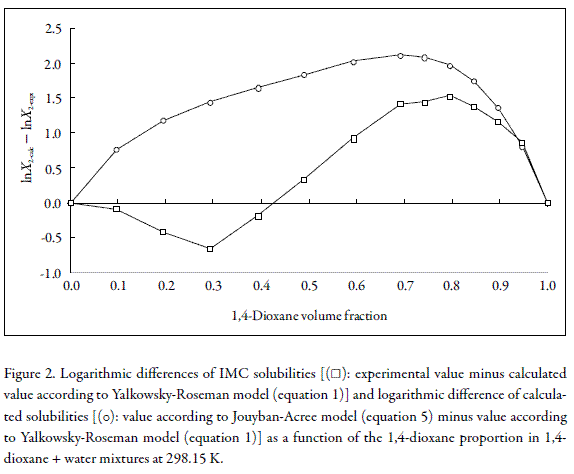
Figure 2 shows that differences obtained in front to Jouyban-Acree model are negative in all cases and dependent on solvent composition being larger in water-rich mixtures.
Thus, experimental solubilities for IMC are lower than those predicted by Equation 5.
Because the equation 5 ( Jouyban-Acree model) is an extension of Equation 1, Figure 2 shows the excess factor of Jouyban-Acree ( J - A factor), which is equivalent to the logarithmic difference between calculated solubilities by using both equations, and it is a global excess solubility function. Besides, Figure 2 shows the logarithmic differences obtained between experimental values of IMC solubility and those calculated by assuming log-linear behavior (Equation 1). This figure also shows the differences obtained in IMC calculated solubilities by using log-linear behavior (Equation 1) and by using Equation 5 ( Jouyban-Acree model) at 298.15 K.
According to Figure 2, IMC exhibits negative and positive deviations with respect to log-linear model and just negative in front to Jouyban-Acree model. It is important to note that IMC does not follow a similar trend to that described by Jouyban-Acree model which assumes positive deviations with respect to logarithmic additivity (log- linear model) in all mixtures. Thus IMC exhibits negative deviations in water-rich mix- tures and positive deviations in 1,4-dioxane-rich mixtures.
The trend exhibited by IMC in Figure 2 is similar to those reported by Rubino and Obeng (18) for the solubility of homologue series of some alkyl p-hydroxybenzoates and p-aminobenzoates in propylene glycol + water cosolvent mixtures. These solutes also exhibited negative deviations in water-rich mixtures and positive in propylene glycol-rich mixtures with respect to log-linear equation.
A possible explanation for negative deviations observed in the drug solubility at low cosolvent proportions could be found in the research reported by Kimura et al. (28), where similar behaviors were found in dissolution enthalpies of 1-methyl-2-pyrrolidinone in ethanol + water mixtures. According to these investigators at low cosolvent proportions the water retains its ability to form ordered structures.
Although alcohols of low molar masses have been considered as polar compounds, Matsumoto et al. (29) based on excess molar enthalpy values have presented some evidence about the influence of the ending methyl group on the water structure formation. The interactions present between alcohols and water could diminish the interactions between water and the drug leading to lower solubility values as expected according to log-linear model.
On the other hand, at high cosolvent concentrations in the mixtures the tridimensional structure of water is lost and therefore the water molecules could be available to interact with the drug molecules. This event would lead to larger solubilities than those expected according to log-linear model (Equation 1). According to the literature another plausible explanation to positive deviations to log-linear equation could be due to possible drug association phenomenon in the saturated solution (18). Nevertheless, in order to verify this event it would be necessary to dispose of any other kind of experimental evidence, such as organic solvent/water drug distribution coefficients at several concentrations and temperatures, or spectroscopic information.
From all topics discussed previously it follows that IMC experimental solubilities present negative deviations in front to those predicted by the Jouyban-Acree model in the 1,4-dioxane + water binary solvent system at all compositions studied. Otherwise, IMC solubility shows negative and positive deviations in front to Yalkowsky-Roseman model. These estimation differences are within 193 % as mean, whereas, Yalkowsky- Roseman model imply differences around 57 % as mean. Thus , neithe r Yalkowsky- Roseman nor Jouyban-Acree models would be useful at industrial level because uncertainties are greater than those usually allowed. This point remarks the great importance to determine experimentally the drug solubility in all the pharmaceutical systems as required.
ACKNOWLEDGEMENTS
The authors thank to the DIB the Universidad Nacional de Colombia (UNC) for the financial support and the Department of Pharmacy of UNC for facilitating us the equipments and installations used in the experimental solubility determinations. Also to Prof. A. Jouyban of Tabriz University of Medical Sciences (Iran) for donating us the bibliographic material required in this investigation.
REFERENCES
1. S. Budavari, M.J. O´Neil, A. Smith, P.E. Heckelman, J.R. Obenchain Jr., J.A.R. Gallipeau, M.A. D´Arecea, "The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals", 13 th ed., Merck & Co., Inc., Whitehouse Station, NJ. 2001. p. 892. [ Links ]
2. R.B. Raffa, Analgesic, antipyretic, and anti-inflammator y drugs , In : "Remington: The Science and Practice of Pharmacy", 21 st ed., edited by A. Gennaro, Lip-pincott Williams & Wilkins, Philadelphia, 2005, pp. 1524-1542. [ Links ]
3. F. Jiménez, F. Martínez, Una estrategia para la selección sistemática de vehículos en el diseño de formas farmacéuticas liquidas homogéneas, Rev. Colomb. Cienc. Quím. Farm., 24, 19-23 (1995). [ Links ]
4. A. Jouyban, "Handbook of Solubility Data for Pharmaceuticals", CRC Press, Taylor & Francis Group, Boca Raton, FL, 2010, pp. 30-58. [ Links ]
5. A. Jouyban, In silico prediction of drug solubility in water-dioxane mixtures using the Jouyban-Acree model, Pharmazie, 62, 46-50 (2007). [ Links ]
6. S.H. Yalkowsky, T.J. Roseman, Solubilization of drugs by cosolvents, In: "Techniques of Solubilization of Drugs", edited by S.H. Yalkowsky, Marcel Dekker, Inc., New York, 1981, pp. 91-134. [ Links ]
7. J.T. Rubino, Cosolvents and cosolvency, In: "Encyclopedia of Pharmaceutical Technology", Vol. 3, edited by J. Swarbrick, J.C. Boylan, Marcel Dekker, Inc., New York, 1988. [ Links ]
8. S.H. Yalkowsky, "Solubility and Solubilization in Aqueous Media", American Chemical Society and Oxford University Press, New York, 1999. [ Links ]
9. A. Martin, P. Bustamante, A.H.C. Chun, "Physical Chemical Principles in the Pharmaceutical Sciences", 4 th ed., Lea & Febiger, Philadelphia, 1993. [ Links ]
10. A. Martin, J. Newburger, A. Adjei, Extended Hildebrand approach: solubility of caffeine in dioxane-water mixtures, J. Pharm. Sci., 69, 659-661 (1980). [ Links ]
11. A. Martin, P.L. Wu, Extended Hildebrand solubility approach: p-Hydroxibenzoic acid in mixtures of dioxane and water, J. Pharm. Sci., 72, 587-592 (1981). [ Links ]
12. P. Bustamante, S. Romero, A. Peña, B. Escalera, A. Reillo, Nonlinear enthalpy-entropy compensation for the solubility of drugs in solvent mixtures: paracetamol, acetanilide and nalidixic acid in dioxane-water, J. Pharm. Sci., 87, 1590-1596 (1998). [ Links ]
13. A. Jouyban-Gharamaleki, L. Valaee, M. Barzegar-Jalali, B.J. Clark, W.E. Acree Jr., Comparison of various cosolvency models for calculating solute solubility in water-cosolvent mixtures, Int. J. Pharm., 177, 93-101 (1999). [ Links ]
14. A. Nokhodchi, J. Shokri, M. Barzegar-Jalali, T. Ghafourian, Prediction of benzodiazepines solubility using different cosolvency models, Farmaco, 57, 555-557 (2002). [ Links ]
15. A. Jouyban, Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures, J. Pharm. Pharmaceut. Sci., 11, 32-58 (2008). [ Links ]
16. K.A. Connors, "Thermodynamics of Pharmaceutical Systems: An Introduction for Students of Pharmacy", Wiley-Interscience, Hoboken NJ, 2002, pp. 61-66. [ Links ]
17. J.T. Rubino, S.H. Yalkowsky, Cosolvency and cosolvent polarity, Pharm. Res., 4, 220-230 (1987). [ Links ]
18. J.T. Rubino, E.K. Obeng, Influence of solute structure on deviations from the log-linear solubility equation in propylene glycol - water mixtures, J. Pharm. Sci., 80, 479-483 (1991). [ Links ]
19. O. Redlich, A.T. Kister, Algebraic representation of thermodynamic properties and the classification of solutions, Ind. Eng. Chem., 40, 345-348 (1948). [ Links ]
20. W.E. Acree Jr., Mathematical representation of thermodynamic properties. Part 2. Derivation of the combined nearly ideal binary solvent (NIBS)/Redlich-Kister mathematical representation from a two-body and three-body interactional mixing mode, Thermochim. Acta, 198, 71-79 (1992). [ Links ]
21. A. Jouyban, N.Y.K. Chew, H.K. Chan, M. Khoubnasabjafari, W.E. Acree Jr., Solubility prediction of salicylic acid in water-ethanol-propylene glycol mixtures using Jouyban-Acree model, Pharmazie, 61, 318-331 (2006). [ Links ]
22. BP 1988, "The British Pharmacopoeia", Her Majesty´s Stationery Offic e, London, Vol. I., 1988, p. 310. [ Links ]
23. M.A. Ruidiaz, F. Martínez, Volumetric properties of the pharmaceutical model cosolvent system 1,4-dioxane + water at several temperatures, Vitae, Rev. Fac. Quím. Farm., 16, 327-337 (2009). [ Links ]
24. E. Vargas, A. Sosnik, F. Martínez, Aplicación del modelo de Jouyban-Acree para la estimación de la solubilidad del naproxeno en mezclas cosolventes etanol + agua, Lat. Am. J. Pharm., 27, 654-660 (2008). [ Links ]
25. E.F. Vargas, H. Barbosa, F. Martínez, Utilidad del modelo de Jouyban-Acree para la estimación de la solubilidad del ibuprofeno y el naproxeno en algunas mezclas cosolventes, Rev. Ing. Invest., 28, 30-36 (2008). [ Links ]
26. M. Gantiva, A. Yurquina, F. Martínez, Desempeño de los modelos de Yalkowsky & Roseman y de Jouyban & Acree en la estimación de la solubilidad del ketoprofeno en mezclas cosolventes etanol + agua, Vitae, Rev. Fac. Quím. Farm., 16, 361-368 (2009). [ Links ]
27. M. Gantiva, E.F. Vargas, M.E. Manzur, A. Yurquina, F. Martínez, Modelos de Yalwosky-Roseman y Jouyban-Acree en la estimación de la solubilidad del ketoprofeno en algunas mezclas cosolventes propilenoglicol + agua, Rev. Colomb. Cienc. Quím. Farm., 38, 156-171 (2009). [ Links ]
28. F. Kimura, S. Murakami, R. Fujishiro, Thermodynamics of aqueous solutions of nonelectrolytes. II. Enthalpies of transfer of 1-methyl-2-pyrrolidinone from water to many aqueous alcohols, J. Solution Chem., 4, 241-247 (1975). [ Links ]
29. Y. Matsumoto, H. Touhara, K. Nakanishi, N. Watanabe, Molar excess enthalpies for water + ethanediol, + 1,2-propanediol, and + 1,3-propanediol at 298.15 K, J. Chem. Thermodyn., 9, 801-805 (1977). [ Links ]














