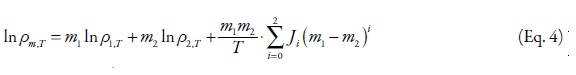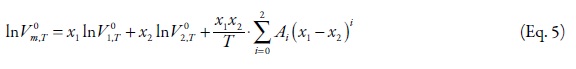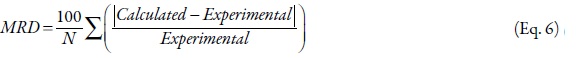Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418
Rev. colomb. cienc. quim. farm. vol.41 no.2 Bogotá July/Dec. 2012
Artículo de investigación científica
Volumetric properties of (PEG 400 + water) and (PEG 400 + ethanol) mixtures at several temperatures and correlation with the Jouyban-Acree model
Propiedades volumétricas de mezclas binarias (PEG 400 + agua) y (PEG 400 + etanol) a diferentes temperaturas y correlación con el modelo Jouyban-Acree
Gerson A. Rodríguez1, Andrés R. Holguín1, Fleming Martínez1*Maryam Khoubnasabjafari2, Abolghasem Jouyban3
1 Grupo de Investigaciones Farmacéutico-Fisicoquímicas, Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia, A.A. 14490, Bogotá, D.C., Colombia.
*Correspondence: E-mail:fmartinezr@unal.edu.co
2Tuberculosis and Lung Disease Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Drug Applied Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
Recibido para evaluación: 15 de octubre de 2012.
Aceptado para publicación: 10 de diciembre de 2012.
SUMMARY
Molar volumes and excess molar volumes were investigated from density values for (PEG 400 + water) and (PEG 400 + ethanol) binary mixtures at temperatures from 283.15 K to 313.15 K. Both systems exhibit negative excess volumes probably due to increased interactions like hydrogen bonding and/or large differences in molar volumes of components. Volume thermal expansion coefficients were also calculated for binary mixtures and pure solvents. The Jouyban-Acree model was used for density and molar volume correlations of the studied mixtures at different temperatures. The mean relative deviations between experimental and calculated density data were 0.1 and 0.5 %, for aqueous and ethanolic mixtures, respectively; whereas, in molar volume data the values were 18.0 and 6.9 %, for aqueous and ethanolic mixtures, respectively. The trained versions of the model for PEG 400 binary solvents could be used to predict the density values of other PEGs with reasonable prediction error employing the density of mono-solvents.
Key words: PEG 400, ethanol, water, binary liquid mixtures, density, excess volume, Jouyban-Acree model.
RESUMEN
En este artículo se calcularon los volúmenes molares y molares de exceso a partir de valores de densidad para los sistemas PEG 400 + agua y PEG 400 + etanol, en todo el intervalo de composición, a temperaturas entre 283,15 y 313,15 K. Los sistemas estudiados presentan volúmenes de exceso negativos probablemente debido a las fuertes interacciones por unión de hidrógeno entre las moléculas de los dos compuestos y a la gran diferencia en los volúmenes molares de los dos componentes puros. También se calcularon los coeficientes de expansión térmica-volumétrica en los solventes puros y las respectivas mezclas. Asimismo, se usó el modelo Jouyban- Acree para correlacionar la densidad y el volumen molar de las mezclas a las distintas temperaturas. Las desviaciones medias relativas en densidad fueron 0,1% y 0,5% para las mezclas acuosas y etanólicas, respectivamente, mientras que las desviaciones obtenidas para volumen molar fueron 18% y 6,9% para las mezclas acuosas y etanólicas, respectivamente. Los modelos obtenidos para las mezclas binarias con PEG 400 pueden usarse para predecir los valores con otros PEG, con un adecuado margen de error, utilizando las densidades de los solventes puros.
Palabras clave:PEG 400, etanol, agua, mezclas líquidas binarias, densidad, volumen de exceso, modelo de Jouyban-Acree.
INTRODUCTION
Water-cosolvent mixtures have been used in pharmacy in order to increase the solubility of drugs poorly soluble in water during the design of homogeneous pharmaceutical dosage forms, such as syrups, elixirs, and concentrate drops, among others (1, 2). Ethanol, 1,2-propanediol (propylene glycol), and polyethylene glycol 400 (PEG 400) are the cosolvents most frequently used in duties associated to products design . In particular, these cosolvents are the more indicated for elaboration of peroral and parenteral medications (3). It is important to note that 1,2-propanediol and PEG 400 are also employed as water evaporation regulators. Even more, ethanol and 1,2-propanediol exhibit good properties as controllers of the microorganisms growth in drug formulations (4). Thus, several examples of pharmaceutical formulations using these cosolvents have been presented by Rubino (1) and Yalkowsky (2).
The mixtures obtained using these cosolvents and water, or even among them for non-aqueous liquid formulations such as soft gelatin capsules, show highly non-ideal behavior due to increased interactions between unlike molecules and large differences in molar volumes of the pure components, which leads to non-additive volumes on mixing (5, 6). For this reason it is necessary to characterize the volumetric behavior of these binary homogeneous mixtures as a function of temperature in order to extend the physicochemical information available for liquid mixtures used in pharmacy. This information is useful to understand the intermolecular interactions present in liquid pharmaceutical systems (6). Also, data related to density of solute free mixture of solvents might be useful in prediction of the density of pharmaceutical substances in mixture of solvents (7).
In this report, the molar volumes and the molar excess molar volumes of the binary systems, a) PEG 400 + water and b) PEG 400 + ethanol, at various temperatures (from 283.15 K to 313.15 K) were calculated according to modified procedures widely exposed in the literature (8-10). This report is a continuation of those presented previously about some volumetric properties for other similar aqueous and non-aqueous binary systems (11-14). It is important to note that in the literature is scarce the volumetric information about these mixtures at low temperatures (below room temperature). Additionally, the Jouyban-Acree model was used to correlate densities and molar volumes with solvent compositions as has been for other binary systems at several temperatures (15-19).
MATERIALS AND METHODS
Reagents
In this investigation dehydrated ethanol (from Merck, Germany) and dehydrated polyethylene glycol 400 (PEG 400 from Dow Chemical Company, U.S.A.) were used and they were in agreement with the quality requirements indicated for medicinal products reported in the American Pharmacopeia USP (18); distilled water with conductivity lower than 2 µS cm-1 was also used. The dehydrated ethanol and PEG 400 employed were maintained over molecular sieve 3 Å (from Merck, Germany) to obtain dry solvents prior to prepare the solvent mixtures.
Cosolvent mixtures preparation
All binary mixtures were prepared in quantities of 40.00 g by mass using a Ohaus Pioneer TM PA214 analytical balance with sensitivity ± 0.1 mg, in concentrations from 0.10 to 0.90 varying in 0.10 in mass fraction of solvent 1 (PEG 400), to study nine mixtures and the two pure solvents. This procedure implies an uncertainty of ± 2 x 10–5 in mole fraction. The mixtures were allowed to stand in Magni Whirl Blue M or Neslab RTE 10 Digital Plus (Thermo Electron Company) water baths at temperatures from 283.15 K to 313.15 K varying in 5.00 ± 0.05 K for at least 30 minutes prior to density determinations.
Density determination
This property was determined using a DMA 45 Anton Paar digital density meter connected to a Neslab RTE 10 Digital Plus (Thermo Electron Company) recirculating thermostatic water bath according to a procedure previously described (20). The equipment was calibrated according to Instruction Manual using air and water at the different temperatures studied (21). From density values, all volumetric properties were calculated as will be indicate in the next section.
RESULTS AND DISCUSSIONS
In Table 1 the composition of both binary mixtures, in mass and mole fractions, in addition to density values at several temperatures are presented. Our density values for PEG 400 [1] + water [2] are in good agreement with those reported in the literature at several temperatures and compositions (22-25). On the other hand, up to the best of our knowledge no density values have reported systematically for PEG 400 [1] + ethanol [2] mixtures in the literature, and therefore no comparison is possible. In all cases, density values decrease almost linearly as the temperature increases and they increase as the PEG 400 proportion increases in the mixtures for both cosolvent systems.
Molar volumes and excess molar volumes
Table 2 summarizes the molar volumes (Vº) for the binary mixtures at all temperatures. Vº values were calculated from Eq. 1.
where, x1 and x2 are the mole fractions and M1 and M2 are the molar masses, for both components respectively, and r is the mixture density.
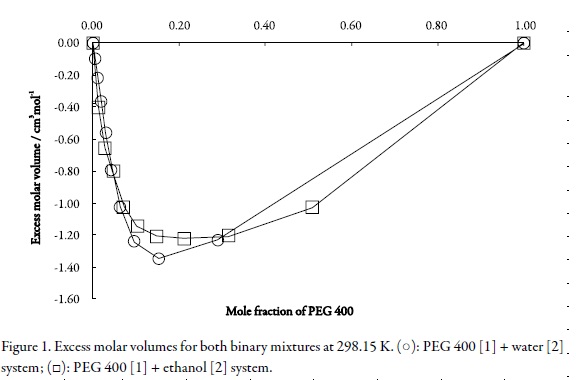
On the other hand, the excess volumes (V0-Exc) calculated from Eq. 2 (where, ρ1 and ρ2 are the densities of pure components) at all temperatures studied are presented in Table 3. This behavior is shown graphically in Figure 1 for both systems at 298.15 K.
Analogous to the behavior obtained for other aqueous and non-aqueous mixtures (11-18), in all cases the excess volumes are negative indicating contraction in volume. Maximum volume contractions were obtained in the mixtures with compositions near to 0.15 or 0.20 in mole fraction of PEG 400 being these values slightly greater for the PEG 400 [1] + water [2] cosolvent system. In this way, according to Fort and Moore (26), a negative excess volume is an indication of strong hetero-molecular interactions in the liquid mixtures and is attributed to charge transfer, dipole-dipole, dipole-induced dipole interactions, and hydrogen bonding between the unlike components, while a positive sign indicates a weak interaction and is attributed to dispersion forces (London interactions), which are operative in all cases.
In the aqueous systems, just as PEG 400 [1] + water [2] is, where the hydrogen bonding predominates, the contraction in volume has been interpreted basically in qualitative terms considering the following events, first: expansion due to depolymerization of water by addition of cosolvent; second: contraction due to free volume difference of unlike molecules; and third: contraction due to hydrogen bond formation between cosolvent and water through –OH---OH bonding (26).
Thus, the large negative values of (V0-Exc) over the free volume contribution indicate the presence of strong specific interactions with predominance of formation of hydrogen bonds between cosolvent and water over the rupture of hydrogen bonding in waterwater. The molecular behavior of PEG 400 [1] + ethanol [2] mixtures could be similar because ethanol is also self-connected although no in the same extent as water is.
For PEG 400 [1] + water [2] mixtures the excess molar volumes becomes less negative as the temperature is raised indicating volume expansion which points out the decrease in the interactions between cosolvent and water molecules with increase in temperature. In contrast, the behavior is opposite for PEG 400 [1] + ethanol [2], i.e. it increases as temperature arises, although the reasons for this result are unclear because of the lack of knowledge about the structure of organic solvents.
Volume thermal expansion
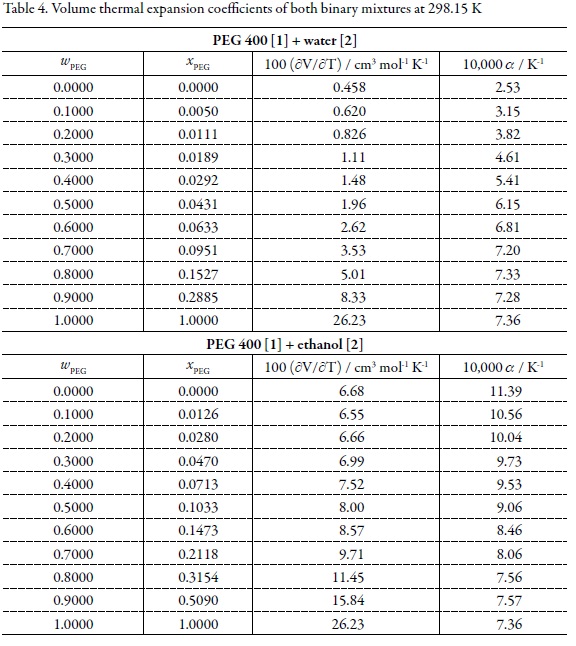
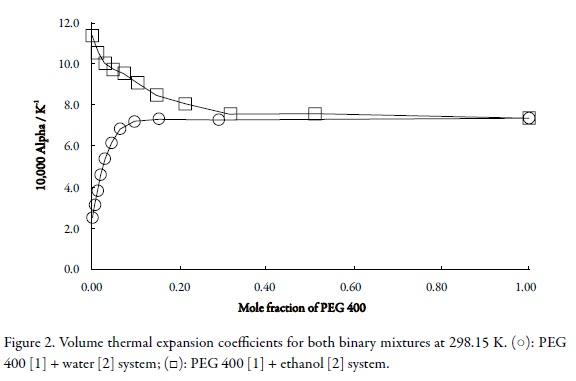
On the other hand, in pharmaceutical and chemical pre-formulation studies, it is very important to predict the variation of physicochemical properties related to pharmaceutical dosage forms, with respect to temperature changes; especially those properties which affect the concentration of active ingredients in the formulations developed. For this reason, the volume thermal expansion coefficients (α) were calculated by means of Eq. 3 (27) using the variation of molar volumes with temperature (Table 2).
Table 4 summarizes the (∂V0/∂T) and a values for pure solvents and binary mixtures. Figure 2 shows the a values at 298.15 K for both cosolvent systems. For all mixtures and pure solvents, linear models were used, in which obtained determination coefficients are greater than 0.999, except for water where quadratic model has been obtained. The a values varied from 2.53 x 10–4 K-1 in water up to 7.36 x 10-4 K-1 in pure PEG 400 and from this value to 1.139 x 10-3 K-1 in neat ethanol. From 0 to 0.030 in mole fraction of PEG 400 the a values increase readily. In a first approach this fact would be explained in terms of water-structure losing by addition of PEG 400. It should be kept in mind that over 0.030 in mole fraction of PEG 400 the most contributing component in mass to all mixtures is just PEG 400, which is also the less polar solvent in these mixtures (1, 2, 28). On the other side, for PEG 400 [1] + ethanol [2] mixtures the behavior is just opposite being a greater for ethanol, although this solvent is more polar than PEG 400.
Data correlation using the Jouyban-Acree model
For binary data analyses, the Jouyban-Acree model was used to correlate the experimental density data of mixed solvents (29):
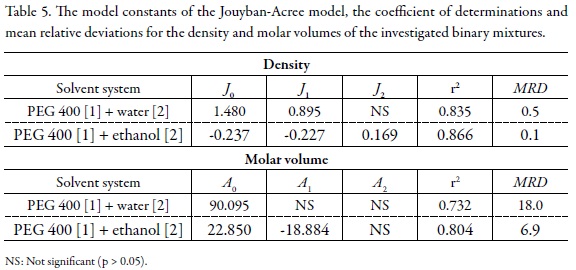
where m1 and m2 are the mass fractions of solvents [1] and [2], ρm,T, ρ1,T, ρ2,T are densities of mixed solvents, and solvents [1] and [2], at different temperatures (T), respectively. The methodology to find the Ji terms was described in previous works (29). The main advantage of the Jouyban-Acree model over other correlation methods such as the one developed by Redlich and Kister (30) is that it includes the effects of temperature in the model constants and provides the possibility of density predictions at other temperatures using interpolation technique, whereas the constants of the Redlich-Kister equation is only valid for one temperature. The interactions between water and/or ethanol with different PEGs are similar and the trained versions of Eq. 4 (for the model constants see Table 5), the densities of their mixtures could be predicted using the density values of the mono-solvents. As examples, the densities of PEG 200 + water, and PEG 600 + water mixtures at 298.2 K taken from earlier works (7, 31) were predicted with the MRDs of 1.0 and 1.1 %, respectively by using the model constants of PEG 400 + water system from this work. The MRDs for the predicted densities of PEG 200 + ethanol and PEG 600 + ethanol were 1.8 and 0.6 %.
An adopted version of Eq. 4 could be used for representing the molar volume data of mixed solvents. The trained version of the model for glycerol + water mixtures at various temperatures is:
where x1, x2 are the mole fractions of solvents 1 and 2, V0m,T, V01,T, V02,T are molar volumes of mixed solvents, and solvents [1] and [2], at different temperatures and Aj are the model constants.
The mean relative deviation (MRD) between experimental and calculated data was calculated using:
where N in is the number of data points in the data set.
The computed model constants of the Jouyban-Acree model, the coefficients of determinations and MRD values for density and molar volume data of the investigated solvent systems are listed in Table 5. Careful examinations of the results reveal that the model describes the densities and molar volumes of the investigated solvent systems and could be recommended for practical applications where the simulation of such data is required in process design.
CONCLUSIONS
This work reports new density values of PEG 400 [1] + ethanol [2] at several temperatures and compositions, whereas it expands widely the experimental volumetric information about PEG 400 [1] + water [2] cosolvent binary systems available nowadays because it includes the behavior at low temperatures commonly found in technological and storage conditions. The reported constants of the Jouyban-Acree model for PEG 400 + water and PEG 400 + ethanol mixtures could be used to predict the densities of other PEGs + water or PEGs + ethanol mixtures employing the density values of the mono-solvents. As mentioned earlier (11-18), this information could be employed in several chemical engineering processes and for the theoretical understanding of the behavior of cosolvent mixtures used in the pharmaceutical industries. Based on the results presented here and those reported in the literature (22-25), it can be concluded that these mixtures clearly show non-ideal behavior. These observations demonstrate clearly that it is necessary to characterize systematically all possible pharmaceutical binary system in order to have complete experimental information about the physical and chemical properties useful in the understanding of all kind of homogeneous liquid pharmaceutical dosage systems.
ACKNOWLEDGMENTS
We thank the DIB of the Universidad Nacional de Colombia (UNC) for the financial support. Additionally we thank the Department of Pharmacy of UNC for facilitating the equipment and laboratories used.
REFERENCIAS
1. J.T. Rubino, Cosolvents and cosolvency, in: "Encyclopedia of Pharmaceutical Technology", Vol 3, edited by J. Swarbrick, J.C. Boylan, Marcel Dekker, New York, 1988, pp. 375-398. [ Links ]
2. S.H. Yalkowsky, "Solubility and Solubilization in Aqueous Media", American Chemical Society and Oxford University Press, New York, 1999, pp. 91-134. [ Links ]
3. D.C. Pérez, C.C. Guevara, C.A. Cárdenas, J.A. Pinzón, H.J. Barbosa, F. Martínez, Solubilidad y volúmenes de desplazamiento del acetaminofén en mezclas binarias formadas por agua, etanol y propilenoglicol a 25.0 °C, Rev. Colomb. Cienc. Quím. Farm., 32, 116-136 (2003). [ Links ]
4. M.E. Aulton, "Pharmaceutics: The Science of Dosage Forms Design", 2nd edition, Churchill Livingstone, London, 2002, pp. 309-322. [ Links ]
5. R. Battino, Volume changes on mixing for binary mixtures of liquids, Chem. Rev., 71, 5-45 (1971). [ Links ]
6. S.J. Rodríguez, D.M. Cristancho, P.C. Neita, E.F. Vargas, F. Martínez, Volumetric properties of the octyl methoxycinnamate + ethyl acetate solvent system at several temperatures, Phys. Chem. Liq., 48, 638-647 (2010). [ Links ]
7. Sh. Soltanpour, A. Jouyban, Solubility of acetaminophen and ibuprofen in binary and ternary mixtures of polyethylene glycol 600, ethanol and water, Chem. Pharm. Bull. (Tokyo), 58, 219-224 (2010). [ Links ]
8. J.A.Salas, J.L. Zurita, M. Katz, Excess molar volumes and excess viscosities of the 1-chlorobutane + pentane + dimethoxyethane ternary system at 298.15 K, J. Argent. Chem. Soc., 90, 61-73 (2002). [ Links ]
9. R.D. Peralta, R. Infante, G. Cortez, R.R. Ramírez, J. Wisniak, Densities and excess volumes of binary mixtures of 1,4-dioxane with either ethyl acrylate, or butyl acrylate, or methyl methacrylate or styrene at T = 298.15 K, J. Chem. Thermodynamics, 35, 239-250 (2003). [ Links ]
10. J.M. Resa, C. González, J.M. Goenaga, M. Iglesias, Temperature dependence of excess molar volumes of ethanol + water + ethyl acetate, J. Solution Chem., 33, 169-198 (2004). [ Links ]
11. J. Jiménez, J. Manrique, F. Martínez, Effect of temperature on some volumetric properties for ethanol + water mixtures, Rev. Colomb. Cienc. Quím. Farm., 33, 145-155 (2004). [ Links ]
12. J. Jiménez, F. Martínez, Study of some volumetric properties of 1,2-propanediol + water mixtures at several temperatures, Rev. Colomb. Cienc. Quím. Farm., 34, 46-57 (2005). [ Links ]
13. J. Jiménez, F. Martínez, Volumetric properties of ethanol + 1,2-propanediol mixtures at different temperatures, Phys. Chem. Liq., 44, 521-530 (2006). [ Links ]
14. G.A. Rodríguez, D.R. Delgado, F. Martínez, M. Khoubnasabjafari, A. Jouyban, Volumetric properties of some pharmaceutical binary mixtures at low temperatures and correlation with the Jouyban-Acree model, Rev. Colomb. Cienc. Quím. Farm., 40, 222-239 (2011). [ Links ]
15. D.M. Cristancho, D.R. Delgado, F. Martínez, M.A.A. Fakhree, A. Jouyban, Volumetric properties of glycerol + water mixtures at several temperatures and correlation with the Jouyban-Acree model, Rev. Colomb. Cienc. Quím. Farm., 40, 92-115 (2011). [ Links ]
16. A.R. Holguín, D.R. Delgado, F. Martínez, M.A.A. Fakhree, A. Jouyban, Study of some volumetric properties of glycerol formal + ethanol mixtures and correlation with the Jouyban-Acree model, Rev. Acad. Colomb. Cienc., 35, 315-328 (2011). [ Links ]
17. D.R. Delgado, F. Martínez, M.A.A. Fakhree, A. Jouyban, Volumetric properties of the glycerol formal + water cosolvent system and correlation with the Jouyban-Acree model, Phys. Chem. Liq., 50, 284-301 (2012). [ Links ]
18. G.A. Rodríguez, D.R. Delgado, F. Martínez, M.A.A. Fakhree, A. Jouyban, Volumetric properties of glycerol formal + propylene glycol mixtures at several temperatures and correlation with the Jouyban-Acree model, J. Solution Chem., 41, 1477-1494 (2012). [ Links ]
19. US Pharmacopeia, 23rd edition, The United States Pharmacopeial Convention, Rockville, MD, 1994. [ Links ]
20. F. Martínez, A. Gómez, C.M. Ávila, Volúmenes molales parciales de transferencia de algunas sulfonamidas desde el agua hasta la mezcla agua-etanol (X = 0.5), Acta Farm Bonaerense, 21, 107-118 (2002). [ Links ]
21. O. Kratky, H. Leopold, H. Stabinger "DMA45 Calculating Digital Density Meter, Instruction Manual", Anton Paar, K.G., Graz, Austria, 1980, pp. 1-12. [ Links ]
22. A. Eliassi, H. Modarress, G.A. Mansoori, Densities of poly(ethylene glycol) + water mixtures in the 298.15-328.15 K temperature range, J. Chem. Eng. Data, 43, 719- 721 (1998). [ Links ]
23. E. Hanke, U. Schulz, U. Kaatze, Molecular interactions in poly(ethylene glycol)–water mixtures at various temperatures: Density and isentropic compressibility study, ChemPhysChem, 8, 553-560 (2007). [ Links ]
24. E. Ayranci, M. Sahin, Interactions of polyethylene glycols with water studied by measurements of density and sound velocity, J. Chem. Thermodynamics, 40, 1200- 1207 (2008). [ Links ]
25. F. Han, J. Zhang, G. Chen, X. Wei, Density, viscosity, and excess properties for aqueous poly(ethylene glycol) solutions from (298.15 to 323.15) K, J. Chem. Eng. Data, 53, 2598-2601 (2008). [ Links ]
26. R.T. Fort, W.R. Moore, Viscosities of binary liquid mixtures, Trans. Farad. Soc., 62, 1112-1119 (1966). [ Links ]
27. J.B. Ott, J. Boerio-Goates, "Chemical Thermodynamics: Advanced Applications", Academic Press, London, 2000, pp. 271-291. [ Links ]
28. A. Barton, "Handbook of Solubility Parameters and Other Cohesion Parameters", 2nd edition, CRC Press, New York, 1991. [ Links ]
29. A. Jouyban, A. Fathi-Azarbayjani, M. Khoubnasabjafari, W.E. Acree, Jr., Mathematical representation of the density of liquid mixtures at various temperatures using Jouyban-Acree model, Indian J. Chem. A, 44, 1553-1560 (2005). [ Links ]
30. O. Redlich, A.T. Kister, Algebraic representation of thermodynamic properties and the classification of solutions, Ind. Eng. Chem., 40, 345-348 (1948). [ Links ]
31. A. Jouyban, Sh. Soltanpour, W.E. Acree Jr., Solubility of acetaminophen and ibuprofen in the mixtures of polyethylene glycol 200 or 400 with ethanol and water and the density of solute-free mixed solvents at 298.2 K, J. Chem. Eng. Data, 55, 5252-5257 (2010). [ Links ]