Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418
Rev. colomb. cienc. quim. farm. vol.43 no.1 Bogotá Jan./June 2014
https://doi.org/10.15446/rcciquifa.v43n1.45462
http://dx.doi.org/10.15446/rcciquifa.v43n1.45462
Artículo de investigación clíca
Assessment of the anticonvulsant activity of pyrazolo[1,5-a][1,3,5]triazines obtained by synthesis
Evaluación de la actividad anticonvulsiva de pirazolo [1,5-a] [1,3,5] triazinas obtenidos por síntesis
1Departament of Chemistry, Universidad de Nariño, Pasto, Colombia, AA. 1175.
2Departament of Chemistry, Universidad del Valle, Cali, Colombia.
Henry Insuasty1, Edison Castro1,2, Juan C. Escobar3, Vanessa Murillo3, Jeanette Rodríguez3, Luis E. Cuca4, Martín Estrada3, Braulio Insuasty2, Mario F Guerrero3.
3Departament of Pharmacy, Faculty of Sciences, National University of Colombia. Bogotá.AA: 14490. Correo electrónico: mfguerrerop@unal.edu.co.
4Departament of Chemistry, Faculty of Sciences, National University of Colombia.
Recibido para evaluación: Enero 19 de 2014
Aceptado para publicación: Mayo 15 de 2014
SUMMARY
Giving that the pyrazolo[1,5-a][1,3,5]triazine system is a potential source of pharmacological agents for treatment of central nervous system disorders this work was aimed to asses anticonvulsant and acute neurotoxic effects of six molecules obtained by synthesis, using experimental models in mice. A series of six pyrazolo[1,5-a][1,3,5]triazines (EAC-21, EAC-31, EAC-33, MH4a, MH4b1 and MH4c) obtained by one-step reaction from S,S-diethyl aroyl-/hetaroylimidodithiocarbonates and 5-aminopyrazoles were screened in vivo (300 mg/kg, v.o.) for their anticonvulsant activity in the maximal electroshock (MES) test in ICR mice and for acute toxicity in the rota-rod and wiring test. The structures of the obtained compounds were unambiguously established by IR, 1H and 13C-NMR spectroscopic techniques, COSY 1H-1H, HSQC and HMBC experiments, mass spectrometry and elemental analyses. Results shown that only MH4b1 showed protection against MES (p<0.05) and was devoid of gross neurotoxicity signs. Therefore, MH4b1 was chosen for additional anticonvulsant screening against MES, pentilenetetrazole, picrotoxin, strychnine and 6 Hz psychomotor seizure model, in a dose dependent manner (50, 150, 300 mg/kg, v.o.). MH4b1 also protected against 6 Hz seizure test (>150 mg/kg, v.o.). These data suggest that MH4b1 could be a source for anticonvulsant pyrazolo[1,5-a][1,3,5]triazine analogs potentially useful against tonic clonic and refractory seizures.
Key words:Pyrazolotriazines, Anticonvulsants, Evaluación Preclínica de Medicamentos, Electroshock, Structure-Activity Relationship.
RESUMEN
Dado el interés que el sistema pirazolo[1,5-a][1,3,5]triazina tiene como fuente potencial para la obtención de agentes farmacológicos para el tratamiento de trastornos del sistema nervioso central, en este trabajo se efectuó el cribado anticonvulsivante y el efecto neurotóxico agudo de seis moléculas derivadas de este sistema, en ratones ICR. La serie de compuestos denominados EAC-21, EAC-31, EAC-33, MH4a, MH4b1 y MH4c, obtenidas por la reacción de S,S-dietil aroil-/heteroilimidoditiocarbonatos y 5-aminopirazoles se evaluó in vivo (300 mg/kg, v.o.) en la prueba de convulsiones inducidas por electrochoque y en las pruebas de actividad neurotóxica aguda del eje rodante y del alambre. La estructura de estos compuestos se determinó por técnicas de infrarrojo, espectroscopia de 1H, 13C-NMR, COSY 1H-1H, experimentos de HSQC y HMBC, espectrometría de masas y análisis elemental. Los resultados mostraron que el compuesto MH4b1 confirió protección frente a las convulsiones inducidas por electrochoque (p<0.05) sin producir signos de neurotoxicidad aguda. Por consiguiente, MH4b1 se escogió para las siguientes pruebas de actividad anticonvulsivante, dosis - respuesta (50, 150, 300 mg/kg, v.o.): electrochoque, pentilenetetrazol, picrotoxin, estricnina y el modelo de convulsión psicomotora de 6 Hz. MH4b1 mostró efectos protectores en función de la dosis frente a las pruebas de convulsión por electrochoque (>150 mg/kg) y convulsión de 6 Hz (300 mg/kg, v.o.). Estos resultados sugieren que MH4b1 podría constituirse en fuente anticonvulsivante del sistema pirazolo[1,5-a][1,3,5]triazina eventualmente útil para el tratamiento de las crisis tónicas clónicas generalizadas y las crisis refractarias.
Palabras clave Pirazolotriazinas, Anticonvulsivantes, Drug screening, Electrochoque, Relación Estructura - Actividad.
INTRODUCTION
The pyrazolo[1,5-a][1,3,5]triazine system has become of great interest in experimental pharmacology in view of its potential utility against a wide variety of biological disorders like chronic obstructive pulmonary disease, psoriasis [1,2], cancer [3, 4, 5], bacteria, fungal and viral infections [6, 7, 8, 9]. Disorders of the central nervous system also are a potential target of pyrazolo[1,5-a][1,3,5]triazine system, including major depression [10, 11] and cognitive disfunction [12], among others.
Epilepsy is the most prevalent neurologic disorder and its morbidity and mortality is high in industrialized and developing countries [13, 14, 15]. Available drug therapy despite the improvements introduced in the last decades, is facing an important side effect profile and insufficient effectiveness in a significant number of patients [16]. Refractactory epilepsy is a challenge key factor in searching for effective anticonvulsant agents [17]. New therapeutic alternatives from natural or synthetic sources may contribute to the treatment of this disease [18].
Insuasty et al developed an efficient method for the preparation of pyrazolo[1,5-a][1,3,5]triazines by the interaction of S,S-diethyl aroyliminodithiocarbonates with 5-amino-3-methylpyrazole to obtain novel 4-aryl-2-ethylthio-7-methylpyrazolo[1,5-a][1,3,5]triazines [19, 20, 21]. In view of potential CNS depressant activities of the pyrazolo[1,5-a]-[1,3,5]-triazine system herein is reported the anticonvulsant profile of the following compounds: EAC-21, EAC-31, EAC-33, MH4a, MH4b1 and MH4c, exposed to electrical and chemical experimental seizures induced in ICR albino mice.
METHODOLOGY
Chemistry
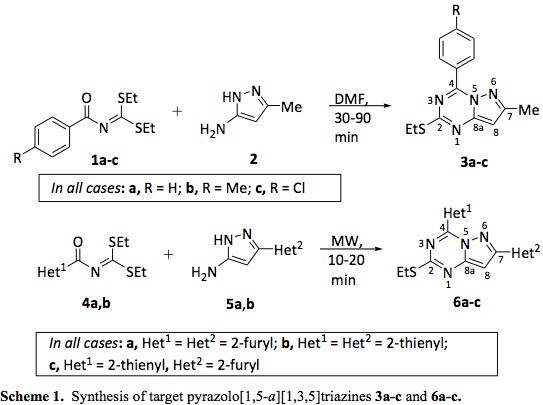
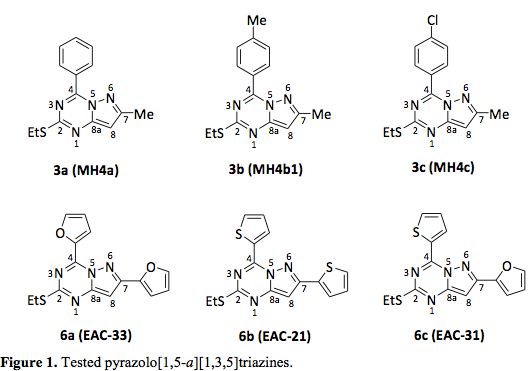
The general synthesis of the desired pyrazolotriazines 3a-c (3a = MH4a, 3b = MH4b1 and 3c = MH4c) and 6a-c (6a = EAC-33, 6b = EAC-21 and 6c = EAC-31) is summarized in scheme 1. Tested pyrazolotriazines are represented in the Figure 1.
General procedure for the synthesis of pyrazolo[1,5-a][1,3,5]triazines 3a-c (3a = MH4a, 3b = MH4b1 and 3c = MH4c).
Pyrazolo[1,5-a][1,3,5]triazines 3a-c were synthetized by heating under reflux a solution of S,S-diethyl aroylimidodithiocarbonates 1a-c (0.003 mol) and 5-amino-3-methylpyrazole 2 (0.003 mol) in dimethylformamide (2 mL), according to the method described by Insuasty et al. (2006). The solid product was precipitated by the addition of cold water to the reaction mixture; the precipitate was collected by ï¬ltration and puriï¬ed by column chromatography on silica gel using a mixture of hexanes/ethyl acetate (4:1) as eluent. Melting point values as well as 1H and 13C nuclear magnetic resonance spectra were consistent with the proposed structures.
Data for 2-ethylthio-7-methyl-4-phenylpyrazolo[1,5-a][1,3,5]triazine (3a = MH4a). Yellow solid, mp 94 °C (90 %). 1H NMR (400 MHz, DMSO): δ 1.36 (t, 3H, CH3), 3.16 (c, 2H, CH2), 6.41 (s, 1H, 8-H), 7.62 (t, 2H, Hm), 7.70 (t, 1H, Hp), 8.57 (d, 2H, Ho). 13C NMR (DMSO): δ 14.3 (CH3), 14.6 (7-CH3), 24.8 (CH2), 94.2 (C-8), 128.4 (Cm), 129.6 (Ci), 130.9 (Co), 133.1 (Cp), 152.0 (C-4), 150.8 (C-8a), 157.4 (C-7), 165.0 (C-2). MS (70 eV) m/z (%): 270 (M+, 100), 255 (28), 139 (20), 70 (18), 51 (19), 39 (16). Anal. Calcd. for C14H14N4S: C, 62.20; H, 5.22; N, 20.72. Found: C, 62.16; H, 5.24; N, 20.67.
Data for 2-ethylthio-7-methyl-4-(4-methylphenyl)pyrazolo[1,5-a][1,3,5]triazine (3b = MH4b1). Green solid, mp 112 °C (63 %). 1H NMR (400 MHz, DMSO): δ 1.40 (t, 3H, CH3), 2.45 (s, 3H, 7-CH3), 3.19 (c, 2H, CH2), 6.33 (s, 1H, 8-H), 7.42 (d, 2H, Hm), 8.58 (d, 2H, Ho). 13C NMR (DMSO): δ 13.6 (CH3), 13.8 (7-CH3), 24.3 (CH2), 93.3 (C-8), 126.5 (Ci), 128.3 (Cm), 130.4 (Co), 143.1 (Cp), 151.3 (C-4), 150.5 (C-8a), 156.8 (C-7), 164.6 (C-2). MS (70 eV) m/z (%): 284 (M+, 100), 269 (22), 251 (57), 139 (27), 118 (24), 70 (15), 39 (15). Anal. Calcd. for C15H16N4S: C, 63.35; H, 5.67; N, 19.70. Found: C, 63.38; H, 5.63; N, 19.69.
Data for 2-ethylthio-4-(4-chlorophenyl)-7-methylpyrazolo[1,5-a][1,3,5]triazine (3c = MH4c). Green solid, mp 104 °C (93 %). 1H NMR (400 MHz, DMSO): δ 1.35 (t, 3H, CH3), 2.40 (s, 3H, 7-CH3), 3.13 (c, 2H, CH2), 6.36 (s, 1H, 8-H), 7.66 (d, 2H, Hm), 8.80 (d, 2H, Ho). 13C NMR (DMSO): δ 14.2 (CH3), 14.5 (7-CH3), 24.8 (CH2), 94.1 (C-8), 128.3 (Cm), 128.5 (Ci), 132.7 (Co), 138.0 (Cp), 151.3 (C-4), 150.7 (C-8a), 157.4 (C-7), 164.8 (C-2). MS (70 eV) m/z (%): 304/306 (M+, 100/42), 289 (23), 273 (20), 271 (52), 139 (45), 138 (32), 137 (18), 110 (20), 81 (20), 70 (17), 51 (17), 39 (18). Anal. Calcd. for C14H13N4SCl: C, 55.17; H, 4.30; N, 18.38. Found: C, 55.21; H, 4.28; N, 18.35.
General procedure for the synthesis of pyrazolo[1,5-a][1,3,5]triazines 6a-c (6a = EAC-33, 6b = EAC-21 and 6c = EAC-31) [21]. A mixture of the appropriate S,S-diethyl hetaroylimidodithiocarbonate 4a,b (0.015 mol) and the corresponding 5-amino-3-hetaryl-1H-pyrazole 5a,b (0.015 mol) was subjected to microwave irradiation in absence of solvent (maximum power 300W during 10-20 min at a temperature in the range of 160 to 180oC), using a focused microwave reactor (CEM discover). When finished (TLC control), the crude product was dissolved in chloroform (3.0 mL) and purified by column chromatography on silica gel, using a mixture of hexanes/ethyl acetate (9:1) as eluent. Note: For describing the spectroscopic data of compounds 6a-c, the heteroaromatic rings (Het1 and Het2) have been denoted by the letters A and B, respectively.
Data for 2-ethylthio-4,7-di(2-furyl)pyrazolo[1,5-a][1,3,5]triazine (6a = EAC-33). Yellow solid, mp 148-149 oC (95 %). 1H NMR (CDCl3): δ 1.48 (t, 3H, CH3), 3.26 (q, 2H, CH2), 6.58 (dd, 1H, HB-4), 6.65 (s, 1H, H-8), 6.77 (dd, 1H, HA-4), 7.04 (d, 1H, HB-5), 7.61 (d, J = 1.8 Hz, 1H, HB-3), 7.86 (d, 1H, HA-5), 8.52 (d, 1H, HA-3). 13C NMR (CDCl3): δ 14.2 (CH3), 25.6 (CH2), 91.2 (C-8), 109.7 (CB-3), 111.9 (CB-5), 113.2 (CB-4), 125.1 (CA-4), 142.9 (CA-2), 143.3 (CB-2), 143.9 (CA-5), 147.9 (C-8a), 148.2 (CA-3), 150.3 (C-7), 150.9 (C-4), 166.9 (C-2). MS (70 eV) m/z (%): 312 (100, M+), 297 (19), 279 (41), 191 (51), 159 (43), 133 (28), 105 (30), 90 (55), 29 (63). Anal. Calcd. for C15H12N4O2S: C, 57.68; H, 3.87; N, 17.94. Found: C, 57.71; H, 3.85; N, 17.92.
Data for 2-ethylthio-4,7-di(2-thienyl)pyrazolo[1,5-a][1,3,5]triazine (6b = EAC-21). Yellow solid, mp 163-164 oC (90 %). 1H NMR (CDCl3): δ 1.50 (t, 3H, CH3), 3.26 (q, 2H, CH2), 6.60 (s, 1H, H-8), 7.16 (dd, 1H, HB-4), 7.31 (dd, 1H, HA-4), 7.45 (dd, 1H, HB-5), 7.63 (dd, 1H, HB-3), 7.84 (dd, 1H, HA-5), 8.94 (dd, 1H, HA-3). 13C NMR (CDCl3): δ 14.4 (CH3), 25.7 (CH2), 90.9 (C-8), 127.1 (CB-3), 127.4 (CB-5), 127.9 (CB-4), 128.5 (CA-4), 132.3 (CA-2), 135.5 (CB-2), 135.9 (CA-5), 137.2 (CA-3), 147.4 (C-8a), 151.4 (C-7), 153.4 (C-4), 166.5 (C-2). MS (70 eV) m/z (%): 344 (41, M+), 311 (16), 270 (16), 207 (18), 178 (35), 134 (24), 110 (48), 70 (61), 45 (54), 29 (100). Anal. Calcd. for C15H12N4S3: C, 52.30; H, 3.51; N, 16.26. Found: C, 52.31; H, 3.52; N, 16.22.
Data for 2-ethylthio-7-(2-furyl)-4-(2-thienyl)pyrazolo[1,5-a][1,3,5] triazine (6c = EAC-31). Yellow solid, mp 108-109 oC (91%). 1H NMR (CDCl3): δ 1.50 (t, 3H, CH3), 3.27 (q, 2H, CH2), 6.60 (dd, 1H, HB-4), 6.64 (s, 1H, H-8), 7.07 (d, 1H, HB-5), 7.32 (t, 1H, HA-4), 7.62 (d, 1H, HB-3), 7.84 (d, 1H, HA-5), 8.97 (d, 1H, HA-3). 13C NMR (CDCl3): δ 14.4 (CH3), 25.7 (CH2), 90.9 (C-8), 109.8 (CB-3), 111.9 (CB-5), 128.5 (CB-4), 132.4 (CA-2), 135.8 (CA-4), 137.3 (CA-5), 143.9 (CA-3), 147.6 (CB-2), 147.9 (C-8a), 150.3 (C-7), 151.2 (C-4), 166.5 (C-2). MS (70 eV) m/z (%): 328 (100, M+), 313 (22), 268 (38), 219 (35), 191 (93), 94 (55), 27 (29). Anal. Calcd. for C15H12N4OS2: C, 54.86; H, 3.68; N, 17.06. Found: C, 54.84; H, 3.65; N, 17.03.
Animals
Male 9-11 week-old ICR albino mice, weighing between 20 and 30g obtained from the Department of Pharmacy, National University of Colombia were used in this study. They were held in conditions of controlled temperature and humidity, photoperiod of 12 hours light /darkness and free access to food and water, except on the test day. The animals were subjected to a fasting period of no more than six hours during the test day in order to not affect the convulsive threshold [22]. 7-8 animals per each dose were used. According to guidelines about anticonvulsant screening and amount of synthesized compounds, an initial 300 mg/kg, p.o. dose was chosen, administered by intragastric cannulation [23]. The maximal electroshock seizure model (MES) in mice was used as first screening assay for comparison to all compounds. Rota rod and wiring tests were applied inmediately before MES test in order to assess the acute neurotoxic effects. The compound that attained greater anticonvulsant response was chosen for the following anticonvulsant tests. All procedures were conducted following the care principles for the management of laboratory animals according to Resolution 008430 of 1993, Title V, Ministry of Social Protection, Colombia. The Ethics Committee of the Faculty of Sciences at the National University of Colombia endorsed this study.
Rota rod: It is a test to detect potentially neurotoxic agents [22]. Groups of seven animals were trained to maintain their balance on an axis of three cm in diameter powered by a small engine at 10 rpm. Mice were previously trained on the rota-rod for 3 min at a speed of 10 rpm. For testing, the animals were placed on the rota-rod one hour after the administration of pyrazole compounds (300 mg/kg, p.o.), valproic acid (reference agent, 300 mg/kg, p.o.) or vehicle (control: hydroxypropylmethylcellulose -HPMC- 0.2%, by volume of 0.01 ml/g, p.o.) before wiring and MES text. A neurotoxic effect was assumed when the animals did not have demonstrated their ability to remain on the revolving rod for at least 1 minute.
Grip strength (wiring test): It is another test to detect potentially neurotoxic agents [23, 24]. Mice were previously exposed to a horizontal thin threat or metallic wire suspended about 30 cm into the air, which they immediately grasp with the forepaws. Mice were released to hang on with their forelimbs. Animals were previously trained to be able to catch the threat with the hind limbs and to climb up within 5 s. For testing, the animals were placed on the wire one hour after the administration of pyrazole compounds (300 mg/kg, p.o.), valproic acid (reference agent, 300 mg/kg, p.o.) or vehicle (HPMC 0.2 %, 0.01 ml/g, p.o.). Animals which were not able to touch the threat with the hind limbs within 5 s or fall off from the threat were considered to be impaired. After this test mice were exposed to MES test.
Maximal electroshock seizures (MES): It is a test to identify potentially effective agents in preventing tonic and clonic seizures. Groups of seven animals were dosed with all pyrazole compounds (300 mg/kg, p.o.), valproic acid (reference agent, 300 mg/kg, p.o.) or vehicle (control: HPMC, 0,2%, p.o.) one hour prior to exposure to an electric shock of 50 mA, 60 Hz and 30 ms through corneal electrodes (E13-51, CI stimulator). A protective effect was assumed when the agent prevented the tonic extension of hind legs at an angle greater than 45° [25]. According the results of this test, MH4b1 was chosen to follow a dose response assay of 50, 150 and 300 mg/kg. p.o., against maximal electroshock seizures (sodium phenytoin: second reference agent, 20 mg/kg, p.o.) and seizures induced by pentylenetetrazole (PTZ), picrotoxin (PIC), strychnine (STR) and 6 Hz discharges. Seizures induced by pentylenetetrazole (PTZ): A test to detect potentially effective agents to prevent absence seizures. Groups of seven animals dosed one hour prior with MH4b1 (50, 150 and 300 mg/ kg, p.o.), clonazepam (reference agent, 0.5 mg / kg, p.o.) or vehicle were administered PTZ (GABA-A antagonist, 85 mg/kg, s.c.). An animal that did not show clonic seizures in its head, back or limbs for more than five seconds over 30 minutes of observation after PTZ administration was considered to be protected [22].
Seizures induced by picrotoxin (PIC): A test used to characterize the anticonvulsant pharmacology of test substances. Groups of seven animals dosed one hour prior with MH4b1 (50, 150 and 300 mg/ kg, p.o.), diazepam (reference agent, 10 mg / kg, p.o.) or vehicle were administered PIC (GABA-A receptor antagonist, 3.5 mg/kg, s.c.). An animal that did not show clonic seizures in its head, back or limbs for more than five seconds over 30 minutes of observation after PIC administration was considered to be protected [26].
Seizures induced by strychnine (STR): Another test used to characterize the anticonvulsant pharmacology of test substances. Groups of seven animals dosed one hour prior with MH4b1 (50, 150 and 300 mg/ kg, p.o.), phenobarbital (reference agent, 20 mg/kg, p.o.) or vehicle were administered STR (glycine receptor antagonist, 2.0 mg/kg, s.c.). An animal that did not show tonic seizures in its head, back or limbs for more than five seconds over 30 minutes of observation after STR administration was considered to be protected [26].
6 Hz psychomotor seizure model: It is a test to identify potentially effective agents for partial refractory epilepsy [27]. Groups of seven animals were dosed with MH4b1 (50, 150 and 300 mg/ kg, p.o.), levetiracetam (reference agent, 35 mg / kg, p.o.) or vehicle one hour prior to exposure to an electric shock of 50 mA, 6 Hz and 3 s through corneal electrodes (E13-51, CI stimulator). A protective effect was assumed when the agent prevented forelimb clonus, twitching of the vibrissae, and Straub-tail [28].
Drugs, reagents and solutions
The following drugs and reagents were used: hydroxypropylmethylcellulose (HPMC, Sigma-Aldrich), valproic acid (VPA, Abbot®), sodium phenytoin (PHE, Parke Davis®), clonazepam (CZP, Roche®), diazepam (DZP), pentylenetetrazole (PTZ), picrotoxin (PIC) and strychnine (STR) (Sigma®). The reference agents (VPA, PHE, CZP and DZP) were prepared in HPMC, 0.2%. The chemical convulsants (PTZ, PIC and STR) were prepared in saline solution 0.9%.
Experimental design and data analysis
The experiments followed a randomized design with 7-8 replicates per treatment. Results are expressed as frequency of protection. The Fisher exact test was applied according to its dichotomous response. Data were analyzed with Excel and Graph Pad® packages. A p value of 0.05 was assumed to be statistically significant.
RESULTS
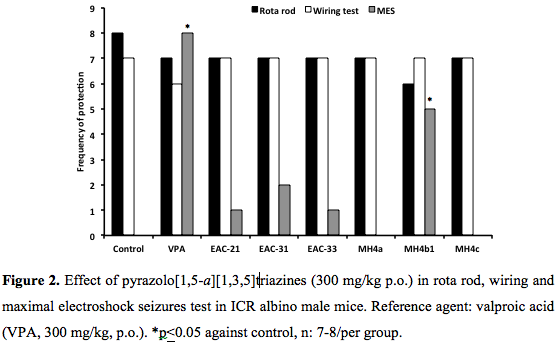
Rotarod and grip strength: The administration of pyrazolo [1,5-α]-1,3,5-triazines: EAC1, EAC-31, EAC-33, MH4a, MH4b1 and MH4c had no gross effect on motor coordination according to results in the rotating shaft and wiring test. The animals remained on the revolving rod for at least 1 minute (6-7/8) and were able to touch the threat with the hind limbs within 5 s or fall off from the threat (7/8), in a similar way than the reference agent, valproic acid (7/8; 6/8) and control (vehicle, 8/8; 7/8) (p>0.05) (Figure 2. ).
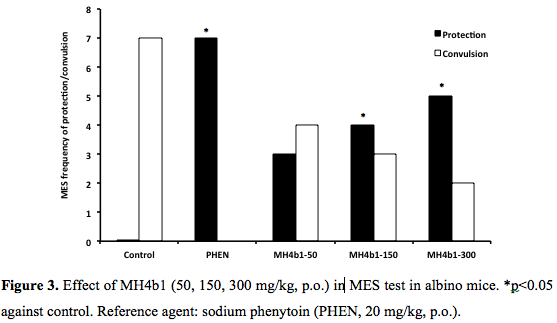
MES seizures: No pyrazolo[1,5-a][1,3,5]triazines but MH4b1 showed protection against maximal electroshock seizures (0/7 versus 5/7) at 300 mg/kg p.o., of screening dose whereas the protection afforded by the reference agent valproic acid (300 mg/kg, p.o.) was completed (8/8, Figure 2). The control group HPMC (0.2%, 0.1 ml/10g) did not prevent any seizure. MH4b1 showed a dose-dependent response in the 50, 150 and 300 mg/kg, p.o., range (3/7, 4/7, 5/7; p<0.05). Sodium phenytoin (reference agent, 20 mg/kg, p.o.) also conferred protection against MES (Figure 3).
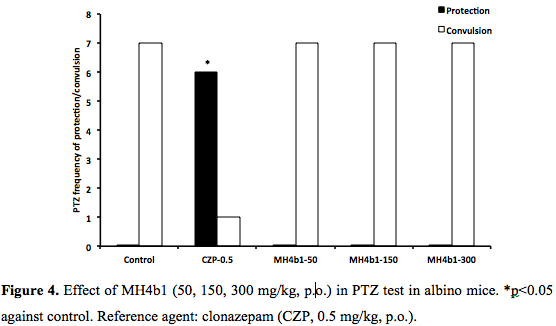
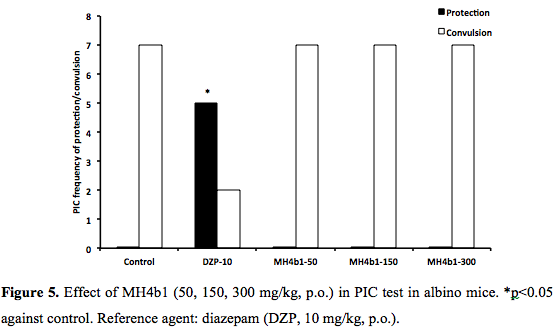
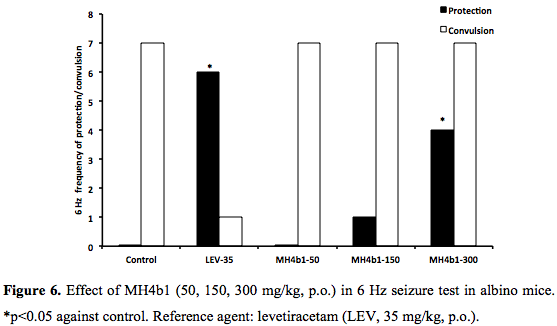
PTZ, PIC, STR and 6 Hz seizures: MH4b1 (50, 150, 300 mg/kg., p.o) did not show any protection against chemical seizures induced by PTZ, PIC and STR (0/7, 0/7, 0/7) at 300 mg/kg p.o., whereas the protection afforded by the reference agent CZP (0.5 mg/kg, p.o.) and diazepam (10 mg/kg, p.o.) was significant (6/8, 5/7 respectively, p<0.05, (Figure 4 and Figure 5). Phenobarbital (20 mg/kg, p.o.) did not protect against STR either (data no shown). The control group HPMC (0.2%, 0.1 ml/10g) did not prevent any seizure. In 6 Hz seizure test, MH4b1 showed a significant effect at 300 mg/kg, p.o., dose (4/7; p<0.05) (Figure 6).DISCUSSION
The results of this study show that among this kind of pyrazolo[1,5-a][1,3,5]triazines, only MH4b1: 2-ethylthio-7-methyl-4-(4-methylphenyl)pyrazolo[1,5-a][1,3,5]triazine exerts significant effects on the maximal electroshock seizures (MES) screening test in mice and 6 Hz psychomotor seizure model whereas it lacks activity against pentylenetetrazole (PTZ), picrotoxine (PIC) and strychnine (STR) seizure tests. Although the compounds EAC-21, EAC-31 y EAC-33 attained protection in one/two animals, these results were not significant. At the same time, MH4b1 would be devoid of acute neurotoxic effects, according to the results in rotarod and grip strength tests. The corollary of these experiments suggests that MH4b1 could have anticonvulsant activity against tonic clonic and refractory partial seizures, a supposition supported by the selective inhibitory effects on MES and 6 Hz tests.
MES, PTZ, PIC and STC tests are among the screening models used to explore possible anticonvulsant effects of a new compound [26]. MES is a useful test to explore possible anticonvulsant activity against tonic clonic seizures, usually related to direct or indirect sodium channels inhibition, although another mechanisms could be implicated [17]. PTZ test is useful to detect agents against myoclonic seizures and with a lesser degree of predictivity, against absence seizures ([17]. This kind of seizures is related to T-type Ca2+ channel activation and/or GABA-A inhibition. Clonic seizures induced by PIC and tonic seizures induced by STR are related to inactivation of chloride ion channel of GABA-A and glycine receptor respectively [29, 30]. Therefore, according to the results of this work, the lack of response to MH4b1 administration in the PTZ, PIC and STC tests leads to the suggestion that the mechanism of action of this agent is not related to either interactions with T-type Ca2+ channel or GABA-A receptor nor glycine receptors whereas some kind of inhibition against sodium channels could be implicated.
In addition, 6 Hz tests are among the screening models used to explore possible anticonvulsant effects against refractory partial epilepsy [27]. 20-40% of the patients with newly diagnosed epilepsy will become refractory to conventional antiepileptic drugs, so there is need to finding drugs effective for the treatment of this kind of epilepsy [31]. Most of conventional antiepileptic drugs (AEDs) are not effective in 6 Hz model. Only few agents like levetiracetam, valproate and novel AEDs such as brivaracetam and retigabine, allow complete protection against the 6-Hz seizures [27]. Interestingly, MH4b1 also gives protection against 6 Hz model, which increases the potential utility of this agent. Given that MH4b1 protects against MES and 6 Hz seizures, additional mechanisms to sodium channels inhibition could be considered, since sodium antagonism could be suffice to protect against MES but not against 6 Hz test. In fact, typical sodium antagonists, such as phenytoin and carbamazepine, display anticonvulsant profile mainly directed to generalized tonic clonic seizures but not to refractory seizures.
The structure of MH4b1 seems to show that the substitution at position 4 of the aromatic ring could play a pivotal role in the anticonvulsant activity of the pyrazolo[1,5-a][1,3,5]triazine. The effect would be greater with an electron donor group.CONCLUSIONS
In conclusion, MH4b1 has in vivo profile in mice consistent with anticonvulsant-like effects against generalized tonic clonic seizures and refractory partial seizures. More research is needed in order to establish its mechanism of action, its structure activity relationship and its pharmacokinetic features that let to MH4b1 attain the central nervous system in enough amount to exert anticonvulsant properties.
ACKNOWLEDGEMENT
This work was conducted with funding from Universidad Nacional de Colombia, Bogotá (VRI/DIB, Project: 16036/14798). Thanks to Animalarium of the Pharmacy Department, Faculty of Sciences, Universidad Nacional de Colombia.CONFLICT OF INTEREST
There were no conflicts of interest in carrying out this work.
REFERENCES
1. P. Raboisson, D. Schultz, C. Muller, J.M. Reimund, G. Pinna, R. Mathieu, P. Bernard, Q.T. Do, R.L. Desjarlais, H. Justiano, C. Lugnier, J.J. Bourguignon, Cyclic nucleotide phosphodiesterase type 4 inhibitors: evaluation of pyrazolo[1,5-a][1,3,5]triazine ring system as an adenine bioisostere, Eur. J. Med. Chem., 43, 816 (2008). [ Links ]
2. A. Gavaldà, R.S. Roberts, Phosphodiesterase-4 inhibitors: a review of current developments (2010 - 2012), Expert Opin. Ther. Pat., 23, 997 (2013). [ Links ]
3. F. Popowycz, C. Schneider, S. Debonis, D.A. Skoufias, F. Kozielski, C.M. Galmarini, B. Joseph, Synthesis and antiproliferative evaluation of pyrazolo[1,5-a][1,3,5]triazine myoseverin derivatives, Bioorg. Med. Chem., 17, 3471 (2009). [ Links ]
4. L. Sun, H. Bera, W.K. Chui, Synthesis of pyrazolo[1,5-a][1,3,5]triazine derivatives as inhibitors of thymidine phosphorylase, Eur. J. Med. Chem., 65, 1 (2013). [ Links ]
5. Z. Nie, C. Perretta, P. Erickson, S. Margosiak, J. Lu, A. Averill, R. Almassy, S. Chu, Structure-based design and synthesis of novel macrocyclic pyrazolo[1,5-a] [1,3,5]triazine compounds as potent inhibitors of protein kinase CK2 and their anticancer activities, Bioorg. Med. Chem. Lett., 18, 619 (2008). [ Links ]
6. S. Bondock, R. Rabie, H.A. Etman, A.A. Fadda, Synthesis and antimicrobial activity of some new heterocycles incorporating antipyrine moiety, Eur. J. Med. Chem., 43, 2122 (2008). [ Links ]
7. D. Mares, C. Romagnoli, E. Andreotti, G. Forlani, S. Guccione, C.B. Vicentini, Emerging antifungal azoles and effects on Magnaporthe grisea, Mycol. Res., 110, 686 (2006). [ Links ]
8. T. Lübbers, P. Angehrn, H. Gmünder, S. Herzig, J. Kulhanek, Design, synthesis, and structure-activity relationship studies of ATP analogues as DNA gyrase inhibitors, Bioorg. Med. Chem. Lett., 10, 821 (2000). [ Links ]
9. K.S. Gudmundsson, B.A. Johns, J. Weatherhead, Pyrazolopyrimidines and pyrazolotriazines with potent activity against herpesviruses, Bioorg. Med. Chem. Lett., 19, 5689 (2009). [ Links ]
10. T. Saito, T. Obitsu, C. Minamoto, T. Sugiura, N. Matsumura, S. Ueno, A. Kishi, S. Katsumata, H. Nakai, M. Toda, Pyrazolo[1,5-a]pyrimidines, triazolo[1,5-a]pyrimidines and their tricyclic derivatives as corticotropin-releasing factor 1 (CRFâ) receptor antagonists, Bioorg. Med. Chem., 19, 5955 (2011). [ Links ]
11. D.M. Nielsen, G.J. Carey, L.H. Gold, Antidepressant-like activity of corticotropin-releasing factor type-1 receptor antagonists in mice, Eur. J. Pharmacol., 499, 135 (2004). [ Links ]
12. P. Jones, J.R. Atack, M.P. Braun, B.P. Cato, M.S. Chambers, D. O'Connor, S.M. Cook, S.C. Hobbs, R. Maxey, H.J. Szekeres, N. Szeto, K.A. Wafford, A.M. MacLeod, Pharmacokinetics and metabolism studies on (3-tert-butyl-7-(5-methylisoxazol-3-yl)-2-(1-methyl-1H-1,2,4-triazol-5-ylmethoxy) pyrazolo[1,5-d][1,2,4]triazine, a functionally selective GABA(A) alpha5 inverse agonist for cognitive dysfunction, Bioorg. Med. Chem. Lett., 16, 872 (2006). [ Links ]
13. G. Pradilla, B. Vesga, F. León-Sarmiento, Grupo Geneco, Estudio neuroepidemiológico nacional (EPINEURO) colombiano, Rev. Panam. Salud Pública., 14, 104 (2003). [ Links ]
14. J. Burneo, J. Tellez-Zenteno, S. Wiebe, Understanding the burden of epilepsy in Latin America: a systematic review of its prevalence and incidence, Epilepsy Res., 66, 63 (2005). [ Links ]
15. Y. Torres, J. Posada, R. Mejia, J. Bareño, G.M. Sierra, L.P. Montoya, "Primer estudio poblacional de salud mental Medellín 2011-2012", CES, Medellin, 2012, p. 113. [ Links ]
16. K. Kobow, S. Auvin, F. Jensen, W. Löscher, I. Mody, H. Potschka, D. Prince, A. Sierra, M. Simonato, A. Pitkänen, A. Nehlig, J.M. Rho. Finding a better drug for epilepsy: antiepileptogenesis targets, Epilepsia, 53, 1868 (2012). [ Links ]
17. M. Bialer, H.S. White, Key factors in the discovery and development of new antiepileptic drugs, Nat. Rev. Drug Discov., 9, 68 (2010). [ Links ]
18. S.C. Schachter, Botanicals and herbs: A traditional approach to treating epilepsy, Neurotherapeutics, 6, 415 (2009). [ Links ]
19. H. Insuasty, M, Estrada, J. Cobo, J.N. Low, C. Glidewell, Isolated molecules in 2-ethylsulfanyl-7-methyl-4-(4-methylphenyl)pyrazolo-[1,5-a] [1,3,5]triazine, Acta Crystallogr, C62, 122 (2006). [ Links ]
20. H. Insuasty.; M. Estrada.; E. Cortés, J. Quiroga, B. Insuasty. R. Abonía, M. Nogueras, J. Cobo, Regioselective synthesis of novel 4-aryl-2-ethylthio-7-methylpyrazolo[1,5-a][1,3,5]triazines, Tetrahedron Lett., 47, 5441 (2006). [ Links ]
21. H. Insuasty, B. Insuasty, E. Castro, J. Quiroga, B. Insuasty, R. Abonía, M. Nogueras. J. Cobo, Three practical approaches for the synthesis of novel 4,7-dihetarylpyrazolo[1,5-a][1,3,5]triazines, Tetrahedron, 68, 9384 (2012). [ Links ]
22. E. Swinyard, J. Woodhead, H. White, M. Franklin, Experimental selection, quantification, and evaluation of anticonvulsants. In: Levy R (Ed) "Antiepilectic Drugs", New York, 1989, p. 85. [ Links ]
23. J.R. Boissier, P. Simon. L´utilisation du test de la traction, (Test de JULOU-COURVOISIER) pour l´etude des psycholeptiques, Therapie, 15, 1170 (1960). [ Links ]
24. A.J. Lapa, C. Souccar, M.T. Lima, T.C.M. Lima, Métodos de evaluación de la actividad farmacológica de plantas medicinales. In: CYTED/CNPq (Ed). Florianópolis, Santa Catarina, 70, (2002). [ Links ]
25. M.M. Castel-Branco, G.L. Alves, I.V. Figueiredo, A.C. Falcão, M.M. Caramona, The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs, Methods Find. Exp. Clin. Pharmacol., 31,101 (2009). [ Links ]
26. W.J. Giardina, Models of Epilepsy: Electroshock and Chemical Induced Convulsions in the Mouse, In: "Current Protocols in Pharmacology", John Wiley & Sons, 5, 221 (2000). [ Links ]
27. W. Löscher, Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs, Seizure, 20, 359 (2011). [ Links ]
28. M.E. Barton, B.D. Klein, H.H. Wolf, H.S. White, Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy, Epilepsy Res., 47, 217 (2001). [ Links ]
29. M.K. Ticku, M. Ban, R.W. Olsen, Binding of [3H]alpha-dihydropicrotoxinin, a gamma-aminobutyric acid synaptic antagonist, to rat brain membranes, Mol. Pharmacol., 14, 391 (1978). [ Links ]
30. A.B. Young, S.H. Snyder, The glycine synaptic receptor: evidence that strychnine binding is associated with the ionic conductance mechanism, Proc. Natl. Acad. Sci., 71, 4002 (1974). [ Links ]
31. J.A. French, Refractory epilepsy: clinical overview, Epilepsia, 48 Suppl. 1, 3 (2007). [ Links ]