Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418
Rev. colomb. cienc. quim. farm. vol.44 no.1 Bogotá Jan./April 2015
https://doi.org/10.15446/rcciquifa.v44n1.54281
Doi: http://dx.doi.org/10.15446/rcciquifa.v44n1.54281
Inhibitory effects of an extract of fruits of Physalis peruviana on some intestinal carbohydrases
Evaluación in vitro del efecto de un extracto de frutos de Physalis peruviana sobre algunas carbohidrasas intestinales
Diana Patricia Rey a
Luis Fernando Ospina b
a Corporación Tecnológica de Bogotá, Bogotá, D. C., Colombia.
b Grupo de Investigación Principios Bioactivos en Plantas Medicinales, Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia, Cra. 30 No. 45-03, Bogotá, D. C., Colombia.
c Grupo de Investigación en Tecnología de Productos Naturales, Tecprona, Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia, Cra. 30 No. 45-03, Bogotá, D. C., Colombia. * Correo electrónico: dmaragonn@unal.edu.co.
Recibido para evaluación: 19 de noviembre de 2014.
Aceptado para publicación: 1° de febrero de 2015.
Summary
Physalis peruviana is an Andean specie whose fruits are eaten as food and also has been reported in Colombian folk medicine for diabetes mellitus treatment. In addition, previous pharmacological studies on diabetic Wistar rats, an extract of P. peruviana fruits has been showed antidiabetic activity. In order to deepen in P. peruviana action mode as antidiabetic, in this investigation were determinate the Inhibitory Concentrations 50 (IC50) of P. peruviana fruits crude extract on alpha glucosidase from S. cerevisiae and from intestinal rat powder, on maltase and alpha amylase enzymes. The kinetic behavior of the extract on each enzyme was also investigated, and the enzyme constant (Km) and maximum rate (Vmax) were determined. Extract of fruits of P. peruviana showed different IC50 for alpha glucosidase from S. cerevisiae and intestinal rat powder, suggesting greater affinity for the enzyme of mammalian source (4114.7 and 3552.7 µg/mL, respectively). For maltase, the IC50 was close to that obtained for alpha glucosidase (4191.0 µg/mL) while for alpha amylase, the extract exhibited the highest inhibition (IC50: 619.9 g/mL). Regarding kinetic behavior, the extract showed competitive inhibition on alpha-glucosidase and maltase, and on the non-competitive type of alpha amylase. These suggest that inhibition of intestinal carbohydrases is one of the modes of action for the antidiabetic activity of fruits of P. peruviana.
Key words: Physalis peruviana, intestinal carbohydrases, alpha glucosidase, alpha amylase, maltase.
Resumen
Physalis peruviana es una especie andina, cuyos frutos además de ser usados como alimento, son empleados en la medicina tradicional para el tratamiento de la diabetes mellitus. Además, estudios farmacológicos previos en ratas Wistar han demostrado actividad antidiabética de extractos de frutos de P. peruviana. Con el fin de profundizar en el modo de acción de la actividad antidiabética de los frutos de P. peruviana, en la presente investigación se determinó la concentración inhibitoria 50 (CI50) del extracto crudo de frutos de P. peruviana, sobre las enzimas alfa glucosidasa obtenida de S. cerevisiae y de polvo intestinal de rata, maltasa y alfa amilasa. El comportamiento cinético del extracto sobre cada una de las enzimas también fue investigado y la constante enzimática (Km) y la velocidad máxima (Vmax) fueron determinadas. El extracto de frutos de P. peruviana, mostró diferentes valores de CI50 para alfa glucosidasa obtenida de S. cerevisiae y para la obtenida de polvo intestinal de rata, sugiriendo una mayor afinidad por la enzima de origen mamífero (4114,7 and 3552,7 µg/mL, respectivamente). Para maltasa la CI50 fue cercana a la obtenida para alfa glucosidasa (4191,0 µg/mL), mientras para alfa amilasa, el extracto presentó la mayor inhibición (CI50: 619,9 g/mL). Respecto al comportamiento cinético, el extracto mostró inhibición de tipo competitiva sobre alfa glucosidasa y maltasa y no competitiva sobre alfa amilasa. Los resultados sugieren que la inhibición de carbohidrasas intestinales es uno de los modos de acción de los frutos de P. peruviana como agente antidiabético.
Palabras clave: Physalis peruviana, carbohidrasas intestinales, alfa glucosidasa, alfa amilasa, maltasa.
Introduction
Diabetes Mellitus Type II is a metabolic disorder characterized by permanent hyperglycemia, which is caused by defects in the secretion or action of insulin. Chronic hyperglycemia is associated with damage, dysfunction and failure of various organs [1]. Currently, there are different treatments for diabetes mellitus type II, including changes in lifestyle, use of oral hypoglycemic drugs or biotechnology products, and pancreatic transplants [2].
However, it has recently been shown that plants play an important role in health care. The World Health Organization has estimated that approximately 80% of the population in emerging countries make regular use of traditional medicine (mostly derived from plants) and primary health care. Traditional medicine also plays an important role in the health care of the remaining 20% of the populations of the emerging economies and some developed countries [3].
The use of plants for medicinal purposes comes from our native communities, where it is passed down from generation to generation; a transmission of knowledge known as ethnomedicine. However, we cannot ignore the scientific and technological advances that have led to a better understanding of the behavior of nature, and it is essential to rigorously investigate the so-called medicinal plants, to determine their safety and effectiveness.
One of the plants used in traditional medicine for the treatment of diabetes mellitus is P. peruviana, commonly known as "uchuva". In addition to its ethnopharmacological use, its fruits have shown in vivo antidiabetic activity, lowering blood glucose levels and some markers of oxidative stress [4].
There are different possible modes of action for antidiabetic drugs; one of them is the inhibition of enzymes necessary for polysaccharide digestion (these enzymes are also called carbohydrases). They are present in the intestinal villi, and participate in the cleavage of polysaccharides, which are converted into monosaccharides (glucose, fructose, galactose). The inhibition of this enzyme slows carbohydrate absorption and decreases postprandial blood glucose levels in both normal and diabetic subjects [2].
In recent years, many researchers have focused on the search for medicinal plants that inhibit carbohydrases [5-7], finding valuable information, particularly on plants with the presence of flavonoids, polyphenols and sugar derivatives [8]. Since fruits of P. peruviana have these secondary metabolites [9-11] the hypoglycemic effect may be due to inhibition of intestinal carbohydrases such as was reported by Pinto et al., 2009 who found that fruits of P. peruviana inhibited the alpha amylase and alpha glucosidase enzymes [11]. Thus, the aim of this study was to deepen in the evaluation of in vitro inhibition of the extract of fruits of P. peruviana on some intestinal carbohydrases and identify the type of inhibition as possible mode for its hypoglycemic action.
Methods and materials
Materials
Alpha-glucosidase derived from Saccharomyces cerevisiae (S. cerevisiae) Sigma/Aldrich G5003, p-nitrophenyl-α-D-glucopyranoside (pNPG) Sigma/Aldrich N1377, Acarbose Sigma/Aldrich A8980, Maltose Sigma/Aldrich 63418, rat intestinal powder (from acetone) Sigma/Aldrich I1630, LiquiColor a commercial kit (Human GLLQ1), porcine pancreatic alpha-amylase (PPA) Sigma/Aldrich A3170, Blue Starch Sigma/ Aldrich S7776, and ethanolic extract of fruits of Physalis peruviana.
Plant material
The P. peruviana fruits were collected in February of 2012, directly by fruit traders from crops located in Granada-Cundinamarca, Colombia. The plant was identified and classified at the Herbario Nacional Colombiano - Universidad Nacional de Colombia and a voucher specimen was deposited in his collection under the code number (COL-574701).
Preparation of the extract
The ethanolic extract of fruits of P. peruviana used was prepared as follows: 400 g of fresh fruits were blended with 300 mL of 96% ethanol. The obtained juice was dryed in a circulant air oven at 40 °C for 24 hours. The resulting dry material was milled and was placed in a 2 L percolator to which 1500 mL of 96% ethanol was subsequently added. After 24 hours, the percolator was opened and the collected extract was concentrated in a rotary evaporator.
Total phenolic content
The total phenolic content were determined by using the Folin-Ciocalteu assay [12]. An aliquot (100 µL) of extracts or standard solution of gallic acid (3.13, 6.25, 12.5, 25, and 50 µg/mL) was added to 2 mL tube. A reagent blank using distilled water was prepared. 100 µL of Folin-Ciocalteu phenol reagent 2N was added to the mixture and shaken. After 5 minutes 200 µL of 7.5% Na2CO3 solution was added to the mixture. The volume was then made up 2 mL with distilled water. After incubation for 120 minutes at room temperature, the absorbance against the reagent blank was determined at 726 nm with an UV-Visible spectrophotometer. Total phenolics content was expressed as mg Gallic Acid Equivalents (mg GA/ g of sample dry weight).
Inhibition of alpha glucosidase derived from S. cerevisiae
The alpha glucosidase inhibition activity was evaluated by measuring the release of p-nitrophenol from p-nitrophenyl-α-D-glucopyranoside (pNPG) following the method proposed by Pistia-Brueggemann, with slight modifications [13]. The assay contained 40 µL of phosphate buffer (0.5 M, pH 6.8); 10 µL of α-glucosidase (1 UI/mL, derived from S. cerevisiae); 10 µL of pNPG (11.3 mM) and P. peruviana extract or acarbose (positive control) in a range of 20 to 10240 µg/mL. The absorbance was read after incubation for 45 minutes at 37 °C in a BioRad microplate spectrophotometer reader at 550 to 415nm. Alpha-glucosidase inhibitory activity was calculated according to equation 1.
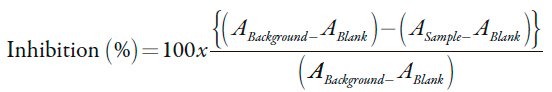 | [1] |
Where ABackground, ABlank and ASample are defined as the absorbance of 100% enzyme activity (only the solvent with the enzyme), the blank (the test sample without the enzyme) and the test sample (with the enzyme) respectively.
Inhibition of alpha glucosidase extracted from rat intestinal powder
The reaction mixture of this test was conducted under the same test conditions as those described in the previous section, changing only the source of alpha-glucosidase, which was obtained from acetone extract of rat intestine, according to previous works [14, 15]. Briefly, 100 mg of rat intestinal powder was suspended in 1 mL of NaCl (0.9%) and sonicated twelve times for 30 seconds at 4 °C. After centrifugation (15000 rpm, 30 min, 4 °C) the supernatant was used for the following tests. The alpha-glucosidase inhibitory activity was calculated according to equation 1.
Inhibition of maltase
Inhibition of maltase was performed by measuring the release of glucose by the Glucose Oxidase-peroxidase method [16, 17] with slight modifications. The assay contained 40 µL of phosphate buffer (0.5 M, pH 6.8); 10 µL of maltase obtained from acetone extract from rat intestine (1 UI/mL); 10 µL of maltose (7.5 mM) and 10 µL of the extract of P. peruviana or Acarbose (positive control) in a range of 20 to 10240 µg/mL. The absorbance was read after incubation at 37 °C for 45 minutes in a spectrophotometer with a microplate reader at 540 nm (BIORAD XMark). The maltase inhibitory activity was calculated according to equation 1.
Inhibition of alpha-amylase
The alpha-amylase inhibition activity was performed following the methodology based on the hydrolysis of starch covalently linked to Remazol Brilliant Blue [18], described by Hansawasdi-Kawabata [19] with slight modifications. For the assay, 200µL of Remazol Brilliant Blue (10 mg/mL) was boiled for 5 min. Next, 100 µL of Tris-HCl buffer (0.05 M, pH 6.9 with calcium chloride 0.01 M), 100 µL of porcine pancreatic alpha-amylase (PPA, 2.1 IU/ml) and 200 µL of extract of P. peruviana or Acarbose (positive control) in a range of 20 to 10240 µg/mL were added. Absorbance was read after incubation at 37 °C for one hour at 595 nm, using a TECAN GENios spectrophotometer. The alpha-amylase inhibitory activity was calculated according to equation 1. All inhibitory concentrations 50 (IC50) were calculated using the GraphPad Prism version 5.00 for Windows software.
Kinetic behavior of P. peruviana extract on the evaluated enzymes
The kinetic behavior of the extract of each enzyme was performed following the methodology described above, except that the substrate concentrations were varied, ranging from 2.2 mM to 16.9 mM. The concentration of the extract was held constant to 5120 µg/mL. The reaction was started by adding the enzyme, and the endpoint was read at 415 nm for alpha-glucosidase, 540 nm to 595 nm for maltase and alphaamylase at time intervals of 5 minutes for 20 min. The results were used to determine the enzyme constant (Km) and maximum rate (Vmax) [20, 21] using a linear regression according to Lineweaver-Burk model, in the GraphPad Prism version 5 for Windows Software.
Statistics analysis
The results were expressed as the arithmetic mean values +/- standard deviation or standard error of the mean, and were analyzed statistically by the Tukey test and analyzed by two-way ANOVA, followed by the Bonferroni test for multiple comparisons. The difference between the sample and the target was considered significant (*) when p < 0.05. The statistical calculations and graphs were processed using GraphPad Prism version 5 for Windows.
Results
Total phenolic content
Previous works have been demonstrated that P. peruviana fruits are rich in phenolic compounds. In this investigation, it was found that employed extract content 6.3 +/- 0.8 mg GAE/g extract, this content is less than that reported by other authors [11]. It can be explained since the solvent used in this investigation (ethanol 96%) is less polar that that the solvent used by Pinto et al. [11] (ethanol 12%).
Inhibitory activity of P. peruviana on alpha glucosidase, maltase and alpha amylase
The inhibition activities of P. peruviana on alpha glucosidase from S. cerevisiae and alpha glucosidase extracted from rat intestinal powder are showed in Figures 1 and 2 respectively. The IC50 values found are reported in the Table 1. It is important to note that the IC50 of acarbose and P. peruviana extract on alpha glucosidase differ according to the source of the enzyme; it is lower when the enzyme is extracted from the intestinal powder.

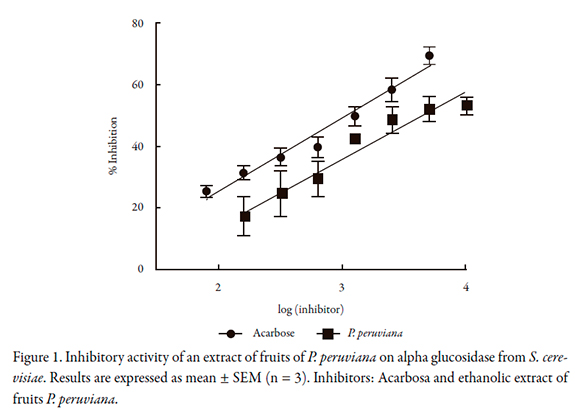
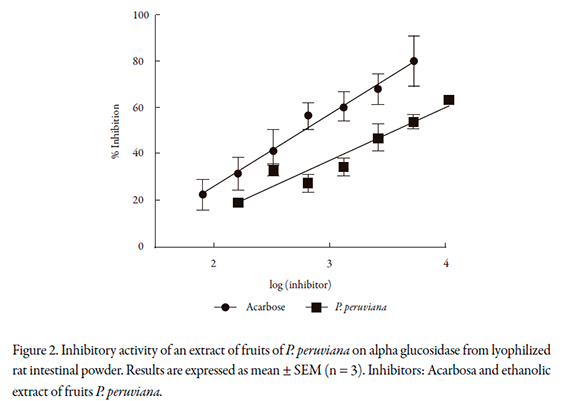
Figure 1. Inhibitory activity of an extract of fruits of P. peruviana on alpha glucosidase from S. cerevisiae. Results are expressed as mean +/- SEM (n = 3). Inhibitors: Acarbosa and ethanolic extract of fruits P. peruviana.The inhibitory activity of the extract of P. peruviana on maltase is shown in Figure 3, and the IC50 value found is shown in Table 1. The IC50 of acarbose on this enzyme is lower than on alpha glucosidase (less than half ) while the IC50 of P. peruviana extract is very close to that found on alpha glucosidase from S. cerevisiae, and slightly higher than those obtained on the extracted alpha glucosidase.
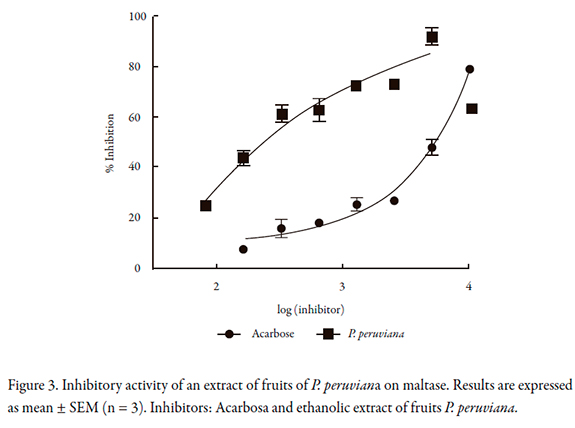
For alpha amylase, the inhibitory activity of P. peruviana is shown in Figure 4. The IC50 value is lower than that found on alpha glucosidase and maltase (Table 1); the lowest IC50 obtained was for P. peruviana.
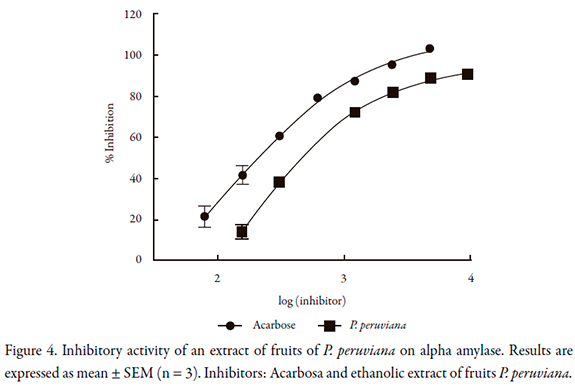
Kinetic behavior of P. peruviana extract on the evaluated enzymes
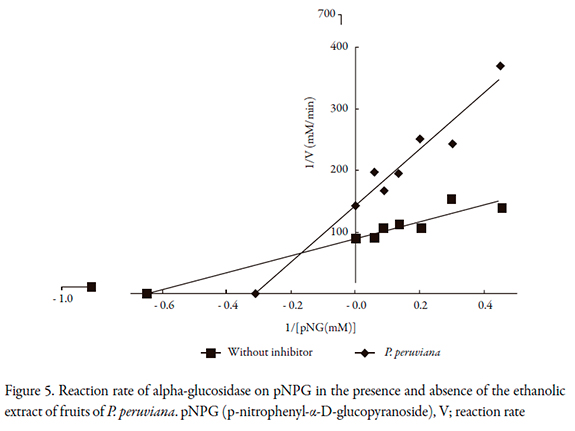
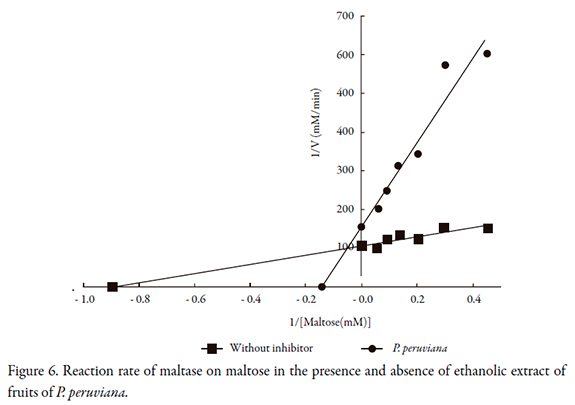
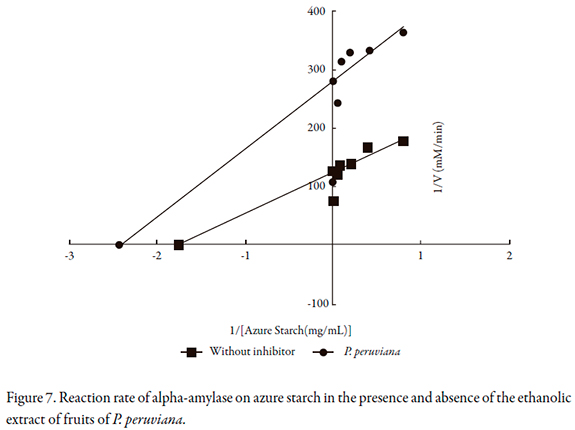
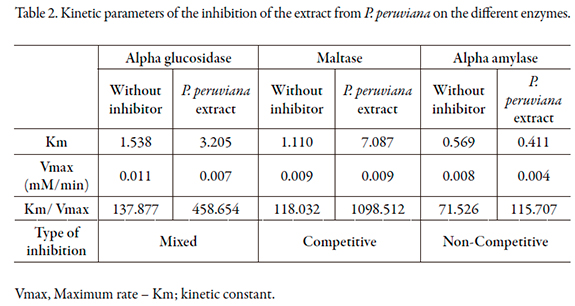
The reaction rates of P. peruviana on alpha-glucosidase, maltase and alpha amylase are shown in Figures 5 to 7, and the kinetic parameters are presented in Table 2. P. peruviana extract has a competitive-type behavior on inhibition of and maltase where the presence of the substrate reduces the inhibitory activity of the extract but not change the maximum rate while it was found a mixed type inhibition on alpha-glucosidase, since the maximum rate (Vmax) is reduced and the enzyme constant (Km) is increased. Regarding alpha amylase, P. peruviana extract exhibited a non-competitive behavior on this enzyme.
Discussion
Inhibitory activity of P. peruviana on alpha glucosidase, maltase and amylase The IC50 found for P. peruviana on alpha glucosidase from S. cerevisiae (4.11mg /mL) was high but similar to the IC50 presented by other extracts of medicinal plants traditionally used as hypoglycemiants. For these plants, IC50 values have been reported ranging from 1.8 µg/mL for Punica granatum extract [22] to 17.2 mg/mL for extract of Andrographis paniculata Nees [23]. On the other hand, in spite of Pinto et al., not reported IC50 values, is possible to note that inhibitions near to 50% was achieve with concentration more than 10 mg/mL of P. peruviana fruits extract [11]. This concentration is higher than the IC50 finding in this investigations, beside the lower content of total phenolic compounds in our extract. These findings suggest that other compounds besides phenols could be responsible of the alpha glucosidase inhibition.
Previous works had reported that the most of the plant extracts have lower IC50 on alpha-glucosidase extracted from S. cerevisiea than on the enzyme extracted from mammalian intestine [24-26]. This is not the case with P. peruviana, which suggests that this extract is more specific for mammalian intestinal enzymes. This fact is important because a plant extract capable of inhibiting the digestion of complex carbohydrates in the human gastrointestinal tract should show inhibition of the enzyme extracted from the mammal, not only the enzyme obtained from microorganisms, since the values reported for inhibition of alpha glucosidase obtained from microorganisms cannot be extrapolated to enzymes from mammalian intestine.
This divergence may be due to the difference in binding site molecular recognition of enzymes. Thus, many inhib itors of alpha glucosidase from S. cerevisiae do not show inhibition on alpha glucosidase extracted from mammals [15, 27]. Some authors report that both acarbose and voglibose have high inhibitory effects of alpha glucosidase in mammals, but no inhibitory activity against alpha glucosidase form S. cerevisiae [26, 27].
For the enzyme maltase on acarbose, a lower IC50 was found than on alpha glucosidase. This is because acarbose binds to the enzyme with higher affinity than the substrate itself [28]. In the case of the ethanolic extract of fruits of P. peruviana, the IC50 was not significantly different from the IC50 on alpha glucosidase. This may be because the extract has a large number of compounds that can bind to various proteins contained in the crude enzyme solution, making its binding less specific. This fact had also been reported in other extracts, such as Stichopus japonicus [29].
For alpha amylase, the IC50 values found were lower, suggesting that the extract of P. peruviana produces a strong inhibition of this enzyme, although P. peruviana IC50 was higher than acarbose IC50 (619.9 and 229.6 µg/mL, respectively). Similar to reported for alpha glucosidase, Pinto et al. found 65% as the maximum inhibition when it was tested P. peruviana extract at concentration of 50 mg/mL, almost 100 times more than the IC50 found in this investigation [11].
The strong inhibition of acarbose on alpha amylase and lack of digestion of starch [30, 31] are related to some side effects such as flatulence and bloating, and diarrhea [15]. Thus, an extract that shows less inhibition on alpha amylase could minimize these side effects [25, 30].
Kinetic behavior of P. peruviana extract on the evaluated enzymes
The kinetic behavior of P. peruviana on maltose is consistent with the results obtained in the inhibition, since the extract behaved very similarly to acarbose, a drug that has a competitive type of inhibition of these enzymes. For alpha glucosidase, it was found a mixed type inhibition such as it was reported for some flavonoids especially quercetin [32], a flavonoid described for Physalis peruviana fruits [11]. However, is important to note that non-competitive behavior is usually preferred, because this prevents the presence or absence of substrate from affecting the binding of the inhibitor to the enzyme, resulting in less interaction with meals [26, 33, 34].
Regarding to alpha amylase, the extract exhibited a non-competitive inhibition that may be related to structural differences due to the origins of the enzymes [35]. Another factor that may explain the combination of competitive and non-competitive inhibition on the evaluated enzymes presented by P. peruviana extract is the fact that the extract presents more than one inhibitor. Due to the presence of polyphenols, such as flavonoids, it is possible for one inhibitor to bind to the active region of alpha-glucosidase and another to bind to the active region of alpha amylase.
Flavonoids or phenolic compounds have been linked to the inhibition of alpha-glucosidase and alpha-amylase [36], and plants with phytochemical studies that have reported the presence of these secondary metabolites, or for which biological studies have demonstrated antioxidant activity, are promising in inhibiting alpha-glucosidase and amylase. For fruits of P. peruviana, the presence of both flavonoids and other phenols has been reported [37], including quercetin, campferol and flavonols [38, 39]. However, the findings of this investigation suggested that not only phenolics compounds but others, could be responsible for the moderate inhibition on alpha glucosidase, and the high inhibition on alpha-amylase.
Conclusions
Ethanolic extract of fruits of P. peruviana has shown different responses to microbial alpha glucosidase (Type I) and alpha glucosidase extracted from rat intestinal powder (Type II). Likewise, ethanol extract of fruits of P. peruviana type shows competitive inhibition on alpha-glucosidase, and on the non-competitive type of alpha amylase.
Based on the results of this investigation, it is suggested that inhibition of intestinal carbohydrases is one of the modes of action by which the fruits of P. peruviana exert hypoglycemic and antidiabetic activity.
Declaration of interest
The authors report no declarations of interest.
References
1. American Diabetes Association (ADA), Diagnosis and classification of diabetes mellitus, Diabetes Care, 35, S64 (2012). [ Links ]
2. A.S. Fauci , E. Braunwald, D.L. Kasper, Diabetes Mellitus, in "Principles of Internal Medicine". 17a Ed. México DF. Mc-Graw Hill Interamericana Editores, Capitulo 338, 2009. [ Links ]
3. R.L. Galvez, Y.I. Kwon, E. Apostolidis, K. Shetty, Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America, Bioresour. Technol., 101, 4676 (2010). [ Links ]
4. A.C. Mora, D.M. Aragón, L.F. Ospina, Effects of Physalis peruviana fruit extract on stress oxidative parameters in streptozotocin-diabetic rats, Lat. Am. J. Pharm., 29, 1132 (2010). [ Links ]
5. K.J. Abesundara, T. Matsui, K. Matsumoto, Alfa-glucosidase inhibitory activity of some Sri Lanka plant extracts, one of which, Cassia auriculata, exerts a strong antihyperglycemic effect in rats comparable to therapeutic drug acarbose, J. Agric. Food Chem., 52, 2541 (2004). [ Links ]
6. S. Önal, S. Timmur, B. Okuttucu, A. Zihnioglu, Inhibition of α-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs, Prep. Biochem. Biotechnol., 35, 29 (2005). [ Links ]
7. L. Yuhao, S. Wen, B. Prasad-Kota, G. Peng, G. Qian-Li, J. Yamahara, B.D. Roufogalis, Punica granatum flower extract, a potent α-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats, J. Ethnopharmacol., 99, 239 (2005). [ Links ]
8. M. Jung, M. Park, H.L. Chul, Y. Kang, E. Seok-Kang, S. Ki-Kim, Antidiabetic agents from medicinal plants, Curr. Med. Chem., 13, 1 (2006). [ Links ]
9. S. Licodiedoff, L.A. Koslowski R.R. Hoffmann, Flavonols and antioxidant activity of Physalis peruviana L. fruit at two maturity stages, Acta Sci. Tech., 35, 393 (2013). [ Links ]
10. S.J. Wu, S.P. Chang, D.L. Lin, S.S. Wang, F.F. Hou, L.T. Ng, Supercritical carbon dioxide extract of Physalis peruviana induced cell cycle arrest and apoptosis in human lung cancer H661 cells, Food Chem. Toxicol., 47, 1132 (2006). [ Links ]
11. M.S. Pinto, L.G. Ranilla, E. Apostolidis, F.M. Lajolo, M.I. Genovese, K. Shetty, Evaluation of antihyperglycemia and antihypertension potential of native Peruvian fruits using in vitro models, J. Med. Food., 12, 278 (2009). [ Links ]
12. C. Soto, E. Caballero, E. Pérez, M.E. Zúñiga, Effect of extraction conditions on total phenolic content and antioxidant capacity of pretreated wild Peumus boldus leaves from Chile. Food Bioprod. Process., 409, 6. (2013). [ Links ]
13. G. Pistia-Brueggeman, R.I. Hollingsworth, A preparation and screening strategy for glycosidase inhibitors, Tetrahedron, 57, 8773 (2001). [ Links ]
14. S.R. Ayinampudi, R. Domala, R. Merugu, S. Bathula, M.R. Janaswamy, New icetexane diterpenes with intestinal α-glucosidase inhibitory and free-radical scavenging activity isolated from Premna tomentosa roots, Fitoterapia, 83, 88 (2012). [ Links ]
15. S.H. Jo, K.S. Ha, K.S. Moon, O.H. Lee, H.D. Jang, Y.I. Kwon, In vitro and in vivo anti-hyperglycemic effects of omija (Schizandra chinensis) Fruit, Int. J. Mol. Sci., 12, 1359 (2011). [ Links ]
16. A. Dahlqvist, Method for assay of intestinal disaccharidases, Anal. Biochem., 7, 18 (1964). [ Links ]
17. S.H. Jo, E.H. Ka, H.S. Lee, E. Apostolidis, H.D. Jang, Y.I. Kwon, Comparison of antioxidant potential and rat intestinal α-glucosidases inhibitory activities of quercetin, rutin, and isoquercetin, Int. J. Applied Res. Nat. Prod., 2, 52 (2009). [ Links ]
18. H. Rinderknecht, P. Wilding, B.J. Haverback, A new method for the determination of alpha-amylase, Experientia, 23, 805 (1967). [ Links ]
19. C. Hansawasdi, J. Kawabata, T. Kasai, α-amylase inhibitor from Roselle (Hibiscus sabdariffa Linn.) tea, Biosci. Biotechnol. Biochem., 64, 1041 (2000). [ Links ]
20. Y.M. Kim, Y.K. Jeong, M.H. Wang, W.Y. Lee, H.I. Rhee, Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia, Nutrition, 21, 756 (2005). [ Links ]
21. L.J. Shai, S.R. Magano, S.L. Lebelo, A.M. Mogale, Inhibitory effects of five medicinal plants on rat alpha-glucosidase: Comparison with their effects on yeast alpha-glucosidase, J. Med. Plants. Res., 5, 2863 (2011). [ Links ]
22. Y. Li, S. Wen, B.P, Kota, G. Peng, G. Qian, J. Yamahara, B.D. Roufogalis, Punica granatum flower extract, a potent α-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats, J. Ethnopharmacol., 99, 239 (2005). [ Links ]
23. R. Subramanian, M.Z. Asmawi, A. Sadikun, In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide, Acta Biochim. Pol., 55, 391 (2008). [ Links ]
24. K.H. Babu, A.K. Tiwari, P.V. Srinivas, A.Z. Ali, B.C. Raju, M. Rao, Yeast and mammalian a-glucosidase inhibitory constituents from Himalayan rhubarb Rheum emodi Wall. ex Meisson, Bioorg. Med. Chem. Lett., 14, 3841 (2004). [ Links ]
25. Y.I. Kwon, E. Apostolidis, K. Shetty, In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension, Bioresour. Technol., 99, 2981 (2008). [ Links ]
26. S.H. Kim, S.H. Jo, Y.I. Kwon, J.K. Hwang, Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model, Int. J. Mol. Sci., 12, 3757 (2011). [ Links ]
27. S. Kumar, V. Kumar, M. Rana, D. Kuma, Enzymes inhibitors from plants: An alternate approach to treat diabetes, Pharmacog. Commun., 2, 18 (2012). [ Links ]
28. G. Mertes, Efficacy and safety of acarbose in the treatment of type 2 diabetes: Data from a 2-year surveillance study, Diabetes Res. Clin. Pract., 40, 63 (1998). [ Links ]
29. H.T. Nguyen, S.M. Kim, Three compounds with potent α-glucosidase inhibitory activity purified from sea cucumber Stichopus japonicus, SPISE, 1, 112 (2009). [ Links ]
30. H. Bischoff, Pharmacology of α-glucosidase inhibition, Eur. J. Clin. Invest., 24, 3 (1994). [ Links ]
31. P. Wongsa, J. Chaiwarit, A. Zamaludien, In vitro screening of phenolic compounds, potential inhibition against a-amylase and a-glucosidase of culinary herbs in Thailand, Food Chem., 131, 964 (2012). [ Links ]
32. K. Tadera, Y. Minami, K. Takamatsu, T. Matsuoka, Inhibition of α-Glucosidase and α-Amylase by flavonoids, J. Nutr. Sci. Vitaminol, 52, 149 (2006). [ Links ]
33. B. Mayur, S. Sandesh, S. Shruti, S. Sung-Yum, Antioxidant and α-glucosidase inhibitory properties of Carpesium abrotanoides L., J. Med. Plants. Res., 4, 1547 (2010). [ Links ]
34. S. Shihabudeen, D.H. Priscilla, K.T. Thirumurugan, Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats Nutrition & Metabolism, 8, 46 (2011). [ Links ]
35. S. Chiba, Molecular mechanism in α-Glucosidase and glucoamylase, Biosci. Biotechnol. Biochem., 61, 1233 (1997). [ Links ]
36. G. Oboh, A.O. Ademiluyi, A.J. Akinyemi, T. Henle, J.A. Saliu, U. Schwarzenbolz, Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro, J. Funct. Foods, 4, 450 (2012). [ Links ]
37. I.S. Cerón, J.C. Higuita, C. Cardona, Capacidad antioxidante y contenido fenólico total de tres frutas cultivadas en la región andina, Vector, 5, 17 (2010). [ Links ]
38. M. Arun, V.V. Asha, Preliminary studies on antihepatotoxic effect of Physalis peruviana Linn. (Solanaceae) against carbon tetrachloride induced acute liver injury in rats, J. Ethnopharmacol., 111, 110 (2007). [ Links ]
39. S.J. Wu, L.T. Ng, Y.M. Huang, D.L. Lin, S.S. Wang, S.N. Huang, C.C. Lin, Antioxidant activities of Physalis peruviana, Biol. Pharm. Bull., 28, 963 (2005). [ Links ]













