Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418
Rev. colomb. cienc. quim. farm. vol.44 no.3 Bogotá Sept./Dec. 2015
https://doi.org/10.15446/rcciquifa.v44n3.56285
DOI: http://dx.doi.org/10.15446/rcciquifa.v44n3.56285
Novel gel-like microemulsion for topical delivery of Amphotericin B
Una nueva microemulsión tipo gel para la aplicación tópica de anfotericina B
Claudia Salerno a
Susana Gorzalczany b
Alicia Arechavala c
Silvia L. Scioscia a
Adriana M. Carlucci a
Carlos Bregni a
a Department of Pharmaceutical Technology, Faculty of Pharmacy and Biochemistry, University of Buenos Aires, Buenos Aires, Junin 956. 6th floor - (1113), Argentina. E-mail: cbregni@gmail.com
b Department of Pharmacology, Faculty of Pharmacy and Biochemistry, University of Buenos Aires, Buenos Aires, Argentina.
c Mycology Unit. Hospital de Infecciosas F. J. Muñiz, CABA, Argentina.
Recibido para evaluación: 27 de abril de 2015.
Aceptado para publicación: 15 de noviembre de 2015.
SummaryThe aim of the present work was to develop a ME for topical delivery of Amphotericin B (AmB). Microemulsions (MEs) are versatile systems to solubilize drugs due to the presence of both a hydrophobic and a hydrophilic region, as well as a distinctive interface composed of surfactant and cosurfactant. MEs have been reported for many advantages for topical application of drugs. Considering that AmB has very low water solubility a screening of surfactants and oils was performed. A gel-like ME system, that can be applied topically without the need for thickeners agents, was selected. AmB was incorporated up to 1 mg/g and remained stable for at least 90 days both at 4 °C and room temperature, so this formulation would be appropriate as a compounding medication. An in vitro skin penetration test was performed, the applied dose penetrated (10.16 +/- 0.01 µg/cm2/h as estimated flux) and remained completely within the skin during the assay; AmB was not detected in the receptor compartment. In vitro antifungal and antileishmanial activity was tested and drug showed proper activity. AmB is a second line drug for the treatment of cutaneous leishmaniasis, but topical dosage forms are still lacking. This system is potentially useful for the treatment of skin infections avoiding drug toxic systemic effects.
Key words: Gel-like microemulsion, amphotericin B, topical delivery, cutaneous leishmaniasis.
Resumen
Se desarrolló una microemulsión (ME) para la aplicación tópica de anfotericina B (AmB). Las ME son sistemas versátiles que facilitan la solubilización de principios activos y, además, presentan muchas ventajas para aplicar fármacos en forma tópica. La AmB posee muy baja solubilidad en agua, por lo cual se realizó una evaluación de su solubilidad en distintos aceites y surfactantes. Se confeccionaron diagramas ternarios de fase y se seleccionó una ME-gel que puede ser aplicada tópicamente sin el agregado de agentes espesantes. Se solubilizó hasta 1 mg/g de AmB y el fármaco permaneció estable durante al menos 90 días a 4 °C y a temperatura ambiente, por lo cual esta formulación sería apropiada como un medicamento magistral. El estudio in vitro de permeación en piel mostró que la dosis aplicada penetró completamente (flujo estimado de difusión 10,16 +/- 0,01 µg/cm2/h) y permaneció retenida en la misma durante el tiempo de estudio sin detectarse AmB en el compartimento receptor. La actividad antifúngica y antileishmania in vitro fue adecuada. La AmB es una droga de segunda línea en el tratamiento de la leishmaniasis cutánea; sin embargo, no se dispone de preparados para uso tópico. Esta formulación sería útil para el tratamiento local de las infecciones de piel, evitando efectos adversos sistémicos.
Palabras clave: microemulsión tipo gel, anfotericina B, aplicación tópica, leishmaniasis cutánea.
Introduction
Microemulsions (MEs) are defined as translucent isotropic thermodynamically stable systems composed of an oil phase, an aqueous phase, and a mixture of a main surfactant and a co-surfactant. MEs can be prepared from a wide variety of oils and surfactants. For some time now, MEs have been extensively studied because of their advantages such as increased solubility of drugs, stability and easy preparation. On account of their versatility and biocompatibility MEs have been investigated for parenteral, oral and topical administration. Several authors have studied MEs potential to improve topical and transdermal administration of drugs in comparison with other conventional vehicles. The increased efficiency of penetration-permeation of the drugs carried into MEs can be attributed to the combined action of composition and structural factors. Due to the presence of hydrophilic and lipophilic domains, it is postulated that MEs can interact with both lipids and proteins of SC; several mechanisms have been proposed to explain the role of MEs for the skin penetration and transdermal passage of drugs. Many hydrophilic and hydrophobic drugs have been incorporated in MEs to increase penetration and skin permeation [1-10].
Although MEs are homogeneous systems in sight, microscopically they are dynamic heterogeneous systems in which the interface spontaneously fluctuates. A surfactant dispersed in an aqueous media would associate in different equilibrium phases, depending on van der Waals forces and the system entropy. During ME formation the increase in interfacial area is very important (in the order of 104 - 105) due to the great number of new droplet. Surfactants generally orientate in order to optimize solvation and reduce free energy, so surfactant-cosurfactant mixture is located at the interface. Three main types of MEs can be formed: o/w or w/o depending whether the internal phase is oil or water, and bicontinuous ME when surfactants form a continuous domain along the oil-water interface with a zero net curvature [10-12].
According to some researchers a gel-like ME is an o/w system where there is a strong hydration of the surfactant hydrophilic chains, which connect themselves through hydrogen bonds resulting in a strong interaction among droplets that modifies ME viscosity [4, 13]. It has also been described that less ordered MEs are more fluid, while those more ordered (cubic and hexagonal phases) show the elastic properties of solids [14, 15]. Another case is that of lecithin, which forms cylindrical or worm-like micelles in MEs with low water content, whose interactions tend to produce a large increase in the viscosity of the system [16, 17]. Gel-like ME systems are useful for topical application without the need of an additional viscosity modifier, also they may enhance drug residence time and regulate drug release in some cases [18].
The polyene macrolid antifungal Amphotericin B (AmB) has a very low solubility in water (1 µg/ml) at physiological pH and is unstable in solution, also AmB causes many adverse effects when administered by parenteral route. Many strategies have been attempted to get over these problems; although some of them have improved AmB therapeutic index and reduced adverse effects, they resulted in expensive medications or higher required doses. AmB is used for invasive infections caused by Candida spp. and Aspergillus spp., and is a second line drug for Leishmania spp. parasites [19, 20].
Cutaneous leishmaniasis (CL) is the most prevalent clinical form of the disease caused by Leishmania spp. CL has been treated so far with sodium stibogluconate and meglumine antimoniate as first line treatment but there is concern about adverse effects such as cardiotoxicity, liver and kidney failure, and the emerging resistance favored by low patient compliance to long parenteral treatments; there is a need for new drugs or more cost-effective therapies with improved formulas. Although CL is usually self-limiting, the goal of the treatment is to prevent disfiguring lessons and the development of more serious forms of disease [21-24]. In some cases of CL drugs were administered locally intralesion; AmB in lipidic formulations with the addition of ethanol was shown to be effective for the local treatment of CL and it was reported the case of a pediatric patient with persistent CL whose lesions healed after a 3-week topical treatment with AmB cholesteryl sulfate with the addition of ethanol 5% [25-27]. Although progress was made in the knowledge of the pathology, we are still in need of greater commitment for the prevention and control of this neglected disease that mainly affects the poorest regions of the world. In early stages of the disease or cases with no risk of systemic complication topical treatment would offer lower toxicity, ease of administration, better patient compliance and lower costs. The aim of the present work was to develop a topical ME to deliver AmB, evaluate stability, in vitro activity and skin penetration.
Materials and methods
Materials
Amphotericin B (AmB) Alpharma, was kindly supplied by Unifarma SA, Argentina; Polyoxyethylene (10) oleyl ether (Brij® 97) and Polyoxyethylene (4) lauryl eter (Brij® 30) were kindly provided by Croda LA; lauryl alcohol 2 OE (Dehydol® LS 2 HN), and oleth-5 (Emulgin® O5) were supplied by Cognis Argentina. Sorbitan monooleate (Span® 80) was purchased from Droguería Saporiti, Argentina; castor oil and Carboxymethyl cellulose sodium (CMC) were purchased Fabriquimica S.R.L., Argentina. Mygliol® 812 (capric-caprylic triglicerides) was provided by Phoenix Laboratory, Argentina. Amphotericin B Deoxycholate Northia® (L: 695), Argentina. Isopropyl myristate (IPM), sweet almond oil, jojoba oil, soya oil, Tween® 80 and all other reagents were of pharmaceutical or analytical grade. Full-thickness skin for percutaneous absorption test was excised from 4-month-old domestic pig ears from a local commercial supplier (Nueva Granja Burzaco; Rauch, Buenos Aires Argentina). Other materials including Candida species and L. brasilensis can be found in the corresponding method sections.
Determination of AmB solubility in surfactants and oil phases
AmB solubility was determined by adding an excess of drug (20 mg) to 10 g of selected oils or surfactants into a glass vial (n = 3). Vials were protected from light and kept under magnetic stirring at room temperature for 96 h. Then, the equilibrated mixtures were centrifuged at 2500 rpm for 15 min and the supernatant was taken and filtered through a 0.45 µm PTFE (Millipore) filter. A 200 µl aliquot was transferred into a 10 ml volumetric flask and volume was adjusted with either a mixture of methanol: chloroform (50:50) or pure methanol according to excipient miscibility or solubility. AmB concentration was determined by UV spectrophotometry (UV-VIS Spectrophotometer Shimadzu UV-260) at 406 nm, calibration curve of AmB in methanol (range 0.5-10 µg/ml; r2 = 0.9995-1.000) and methanol: chloroform (range 1.0-20 µg/ml; r2= 0.9910). Blanks were prepared for the same dilutions of each oil or surfactant in methanol or solvents mixture, as appropriate.
Pseudoternary phase diagrams
Pseudoternary phase diagrams were constructed to determine ME areas for the following surfactant: cosurfactant (S+CoS) ratios (w/w) 1:1; 1:2; 1:3; 2:1 and 3:1. The surfactant was selected on results of the solubility study and ethanol was included as cosurfactant. Mixtures were added to IPM as oily phase for the ratios: (S+CoS:IPM): 9:1; 8:2; 7:3; 6:4; 5:5; 4:6; 3:7; 2:8; 1:9. Samples were prepared at room temperature. The appropriate amount of reagents was weighed and mixed gently. Then, distilled water was added dropwise, with mild magnetic stirring until visual change of an isotropic transparent to opalescent - turbidity transition. Visual observation was made and recorded as transparent isotropic fluid o/w ME, transparent isotropic o/w gel (gel-like ME) or milky emulsion. We searched for o/w ME areas (isotropic and fluid) and o/w gel-like ME (isotropic gel). Limits for each phase were established by visual observation of transition transparency-turbidity; there was no intention to identify other phases.
Preparation of AmB loaded gel-like ME
Gel-like ME was prepared by first mixing surfactant with cosurfactant and then this mixture was added to IPM in a water bath at 40 °C, with mild stirring. Distilled water was slowly added dropwise with continuous mixing. Then sample was removed from the water bath and subsequently AmB was added. The preparation was kept protected from light under mild magnetic stirring until total dissolution of AmB.
Amphotericin B concentration and stability
AmB-loaded gel-like ME was centrifuged 30 min at 2500 rpm to evaluate signs of separation or drug precipitation. Drug concentration in the selected gel-like ME system was determined at the time of preparation; about 100 mg of the gel-like ME was exactly weight and diluted to 10.0 ml with methanol. The sample was stirred until complete dissolution of gel-like ME. AmB concentration was determined by UV spectroscopy (see above).
For stability study samples were stored at room temperature, at 4 °C and 40 °C. At fixed intervals samples were observed for signs of separation or drug precipitation and an aliquot of about 100 mg was exactly weight and put into a glass vial with 10.0 ml of methanol. Sample was left under magnetic stirring for complete dissolution and AmB was determined by HPLC (Shimadzu SPD-10 A vp Uv/Vis Detector). A Microbore C18 (150 * 2.0 mm, 3 µm) Waters Spherisorb® S3 ODS2 column was used with an isocratic mobile phase composed of acetonitrile: disodium edetate 2.5 mM (45:55) [28]. The injection volume was 20 µl and the flow rate was 0.4 ml/min. UV detection was set at 382 nm and retention time was 4.0 +/- 0.1. Linearity curve was determined for the range 0.06-1.60 µg/ml (r2 = 0.9989). External standard was also used during the measurements.
Gel-like ME characterization
Phase-contrast and polarized light microscopy
Phase-contrast and polarized light microscopy was used to microscopic observation of the gel-like ME. Observations were made at 200X magnification using an Axioskop 2 Plus, Zeiss, Germany, equipped with a Sony Exwave HAD video camera to collect digital images (768 * 494 pixels). A small quantity of sample was placed on the on the glass-slide and covered with a cover slip without any additional treatment. The glassslides were previously cleaned with ethanol and acetone.
Refractive index
Refractive index was measured with an Officine Galileo Refractometer (E0 = -0.0002) at room temperature without any treatment of samples. Determinations were made at initial time (48 h after preparation), 1 and 3 months for samples stored at room temperature and at 4 °C. Refractive indexes of distilled water and glycerin were also determined at each control time as references. Results are given as mean +/- SD (n = 3). Conductivity Conductivity was determined for the blank and AmB-loaded gel-like ME with no additional treatment at initial time (48 h after preparation), 1 and 3 months using a thermo-conductimeter Antares IV; Parsec SA, Argentina. Results are given as mean +/- SD (n = 3).
pH determination
Sample pH was determined at 25 °C with a pH-meter TPA III Altronix. Before measurement samples were diluted 1/10 with distilled water adjusted to neutral pH 7.10 +/- 0.02. Results are given as mean +/- SD (n = 3).
Viscosity and Rheology
Rheological behavior of Amb-loaded gel-like ME was evaluated. Samples stored at room temperature and 4 °C were assayed after a week of preparation in order to evaluate if storage conditions affects the gel-like structure and viscosity, and also because it is recommended to store AmB preparations in the refrigerator. A rotational viscometer was employed (Brookfield PVT synchro-Lectric viscometer). Viscosity was measured through the rotation speed of the spindle number 6 immersed in the sample. The test was carried out at 25 +/- 1 °C and the spindle was rotated at 0.5 rpm. The readings were made after ten minutes. The rheological behavior was evaluated by increasing velocity (0.5- 1-2.5-10 and 20 rpm), readings were made after 5 min at each frequency.
In vitro skin penetration-permeation study
In vitro skin penetration-permeation experiments were performed on Franz diffusion cells using pig ear skin. Pig ears were obtained from a local pork slaughterhouse for human feeding. However, approval has been obtained from the Ethical Committee for the Care and Use of Laboratory Animals of the Faculty of Pharmacy and Biochemistry, University of Buenos Aires (N° 170511-2). Skin was excised from 4-month-old domestic pig ears, obtained from a local commercial supplier. The pig ears were cleaned under running water immediately after excision. The hair from the outer region of the ears was removed and then the skin was carefully separated from cartilage using a scalpel. Subsequently, adipose subcutaneous tissue was removed; full-thickness skin was used with a surface area of 3.14 cm2, thickness of 1 mm for all the samples was controlled with a vernier. After being dried with a tissue, the skin was immediately mounted on the diffusion cells or frozen at -20 °C for a maximum period of 4 weeks [29, 30].
The skin was placed horizontally on Franz diffusion cells, between the donor and receptor compartments. Sink conditions were obtained in the receptor compartment by phosphate-buffered saline (PBS; pH 7.4) with 1% sodium lauryl sulfate (PBS-LSNa); solubility in receptor media was previously determined. The volume of receptor fluid was 15 ml. The receptor compartment was continuously homogenized using a stirring magnetic bar and the temperature was kept at 32 °C using a water circulation system. The skin mounted in the cell was allowed to rest for an hour in contact with receptor media before the application of the samples. Gel-like ME loaded with AmB 1 mg/g and AmB Deoxycholate hydrogel were assayed simultaneously. For the hydrogel preparation AmB Deoxycholate marketed formulation was reconstituted with distilled water, according to instructions, and thickened with CMC 2%. Experiments were performed applying 200 µg of AmB as dose for both formulations.
Serial sampling (6 ml) was performed after 0.5, 1, 2, 4 and 6 h, and fresh receptor liquid was added to receptor compartment in order to replace the buffer. Aliquots were filtered by 0.45 µm nylon filter (CAMEO) and analyzed by HPLC as described above; retention time for samples in PBS-LSNa was 3.4 +/- 0.0 min.
After the experiment, the remnant of the dosage form on the skin was put in a glass vial, skin was washed twice with methanol to recover any rest of the applied dose and the used spatula was also washed so as to collect the dosage form completely. The final volume was adjusted with methanol and the mixture was stirred at 2,500 rpm for 1 h. The solution was filtered and AmB was quantified by HPLC (method was previously assayed for drug recovery). Skin drug penetration, considered as the amount retained within the skin, was calculated by difference between the total dose applied and the sum of the amount that permeated plus the amount present in the remnant sample over the skin. After cleaning the skin as mentioned above stratum corneum (SC) was separated by stripping method [31].
Adhesive tape 3 M Scotch was used to remove SC layers, consecutive adhesive tape strips 1-7 and 8-12 were put in separate vials containing 10.0 ml of methanol and were left under magnetic agitation at room temperature for 24 h and then vortexed for 1 min; after that an aliquot was taken and filtered by 0.45 µm before analyzing. The remaining stripped skin (viable epidermis and dermis; VED) was minced and placed into a vial containing 10.0 ml methanol and treated with an Ultraturrax® T25 Basic (IKA Labortechnik, Germany) with an ice bath, 2 min at 19000. Samples were then left in a shaker at room temperature overnight to ensure adequate extraction of the drug from the skin; after that time samples were centrifugated at 2,500 rpm and supernatants were filtered by 0.45 µm nylon filter and presence of AmB was determined by HPLC.
In vitro antifungal activity
The minimal inhibitory concentration (MIC) of AmB-loaded gel-like ME was determined according to M27-A3 CLSI method for several strains of Candida and different patient isolates [32]. A suspension of Candida (1 * 106 UFC/ml) was incubated with 100 ml of solutions with different concentrations of the antifungal in DMSO ([32] 0.06 mg/ml) or the corresponding dilutions of gel-like ME in a 96-well plate at 35 °C for 48 h. Visual readings were performed after 24 and 48 h. AmB deoxycholate from market (Anfotericina B Northia®, Argentina), AmB standard used as reference in the microbiological lab and AmB raw material used for the preparation of gel-like ME were tested as controls. The MIC was defined as the lowest drug concentration inhibiting clearly visible growth of the fungi. 100 ml of RPMI-solvent without AmB and 200 ml of RPMI-solvent were placed in wells for growth control and for sterility control, respectively. Blank gel-like ME was tested as negative control.
In vitro antileishmania activity
(3H) thymidine incorporation assay was performed on L. brasiliensis promastigotes (M2903 strain). Exponentially growing parasites were centrifuged at 1200 g for 15 min, adjusted to a cell density of 103-107 parasites/ml in fresh LIT medium supplemented with 10% decomplemented fetal bovine serum (Gibco), 100 U/ml penicillin and 100 µg/ml streptomycin. Parasites were seeded in a 96-well microplate (150 µl per well) in presence of 1 µCi (3H) thymidine (3H-T; Perkin Elmer) and allowed to grow incorporating 3H-T during 16 h. Counts per min (cpm) of triplicate cultures were measured by an automated liquid scintillation counter (Packard, Downers Grove, IL). Parasites drug susceptibility was assayed for dilutions (ethanol: water) of AmB loaded gel-like ME and AmB Deoxycholate as reference during 72 h. For the last 16 h of culture, cells were pulsed with 1 µCi per well 3H-T. Medium and ethanol:water were assayed as control of 0% of parasites inhibition [33].
Skin irritation test
Albino male rabbits weighing 1.8-2 kg were used. The experiments were carried out taking into account international guiding principles and local regulations concerning to the care and use of laboratory animals for biomedical research. The experiments were approved by the local Ethics Committee (Exp-FyB: 00747495/2012). The animals had free access to a standard commercial diet and water ad libitum and were kept in room maintained at 22 +/- 1 °C with a 12-h light/dark cycle.
The irritancy of formulation was determined based on the method described by Draize et al. The backs of six animals were clipped free of hair 24 h before the beginning of the assay. Gel-like ME loaded with AmB 1 mg/g was tested on two one inch square sites on the same animal; one site was intact and one was abraded in such a way that the stratum corneum was opened but without bleeding. Formulations were tested undiluted (0.5 g). Each test site was covered with two layers of one inch square surgical gauze secured in place with tape. The entire trunk of the animal was then wrapped with rubberized cloth in order to retard evaporation and hold the patches in one position. 24 h after application, the wrappings were removed and the test sites evaluated for erythema and edema. Evaluations of abraded and intact sites were separately recorded. Test sites were evaluated again 48 and 72 h after application by the same procedure. Maximal score was set in 8. The criterion for skin irritation intensity was assumed as follows: < 0.5 no irritation, 0.5-3 slight irritation and > 6 severe irritation [34, 35].
Statistical Analysis
Data were analyzed using software MS Excel 2007 Data Analysis Add-In programme. T-Student test was performed to see any significant difference (p < 0.05).
Results and discussion
AmB solubility in surfactants and oil phases
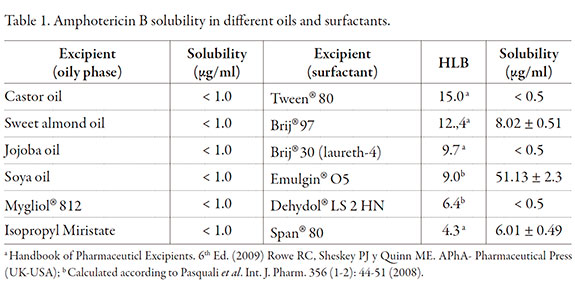
MEs are an interesting system to solubilize poor water soluble drugs so AmB solubility was assayed in several surfactants and oils. Table 1 shows AmB solubility in oil phases and surfactants. AmB proved to be poor soluble in the assayed oils. Among surfactants Emulgin®O5, a polyoxyethylene oleyl eter, turned out to have the best solubilizing capacity.
Phase diagrams and gel-like ME selection
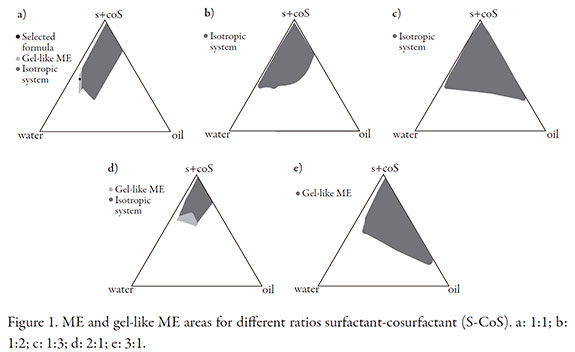
In the search for an extemporaneous formulation with better drug stability we made the attempt to solubilize AmB in ternary mixtures of oils and surfactants studied as potential SMEDDS (self micro-emulsifying drug delivery system) to form ME by water addition. However, the assayed mixtures were not able to significantly enhance drug solubilization. Therefore we searched for ME containing Emulgin® O5, the surfactant in which AmB showed greatest solubility. Ethanol was selected as cosurfactant because as previously mentioned some studies reported that AmB formulations mixed with ethanol were effective for the local treatment of CL. Phase diagrams were constructed with Emulgin® O5, ethanol and IPM was selected as oil phase. Figure 1 shows ME and gel-like ME areas for different ratios S-CoS.
Soy oil was also tested as oily phase but MEs containing this oil were not able to significantly enhance AmB solubility and they had low stability; glyceryin, propylene glycol and diethyleneglycol monoethyl eter (Transcutol® P) were also assayed as cosurfactant but they did not form stable MEs with acceptable surfactant concentration (data not shown).
A gel-like ME system (so called like-gel ME) was selected because it of its suitability to be applied on the skin without the need of additional viscosity modifiers. The ratio surfactant: cosurfactant was set 1:1 in order to use the formulation with less surfactant content for low skin irritancy. Among ME areas found, w/o ME were not selected because of the low oil solubility of AmB and because they are also less comfortable for topical application than o/w ME. An o/w gel-like ME (IPM 5%, Emulgin® O5 20%, Ethanol 20% and distilled water qs 100%; Figure 1a) was selected as optimum candidate for further study as it was the one that remained more stable during macroscopic observation and the one that allowed greater drug loading during preliminary evaluation of possible compositions. When preparing MEs temperature and mixing speed can affect the formation of an isotropic phase. For the gel-like ME is very important to keep mild stirring while adding slowly the aqueous phase, otherwise high stirring velocity would lead to hazy system because of the entrapment of air, and the translucent system is reached after leaving the system to equilibrate.
Ternary mixtures were not able to enhance AmB solubilization; therefore, AmB was incorporated to the whole prepared gel-like ME. It is to remark that a greater quantity of AmB solubilized in the complete ME than in the surfactants mixture, this fact may be explained by the amphiphilic and amphoteric nature of the drug (pKa -COOH 5.7 y pKa -NH2 10.0), so drug would locate in the interface with the surfactant monomers, thus leading to a greater solubility in the complete ME containing the aqueous phase.
Similar results were reported for lecithin based formulations and other amphiphilic association systems for AmB [36-38].
Amphotericin B concentration and stability
Blank gel-like ME and AmB loaded samples showed neither changes nor precipitated drug after centrifugation test. The selected formula solubilized up to 1 mg/g of AmB (1.00 +/- 0.06 mg/g) at time 0. Samples stored at 4 °C were stable for at least 3 months, AmB concentration > 90% (97.96 +/- 5.99%) and no macroscopic change was observed, whereas the drug content for samples at room temperature was 94.97 +/- 7.12% after that period of time and the gel structure became more fluid. On the contrary AmB concentration in samples stored at 40 °C was 88.69 +/- 4.08% after one month of storage and also drug precipitated was observed at the bottom of vials.
Since AmB desoxycholate forms weak micelles with poor stability after reconstitution, reconstituted preparation has to be used within a week therefore the use of AmB desoxycholate marketed formulations for topical application as was reported elsewhere would be less convenient; the type of ME presented here may offer an alternative for topical treatment and according to stability AmB loaded gel-like ME could be prepared as a compounding formulation.
Gel-like ME characterization
Macroscopic aspect of gel-like ME is shown in Figure 2. Figure 3 shows microscopy images of loaded gel-like ME, blank samples showed no difference. Even though the system is macroscopically isotropic, round structures could be observed due to birrefringence when visualized by phase-contrast and polarized light microscopy.


The structural characteristics and mechanical behavior depend on the composition of the system. It is known that less ordered ME usually have low viscosity, whereas the more ordered hexagonal o cubic phases show viscoelastic properties of a jelly-like phase [15, 36]. According to some authors, ME viscosity may be due to the oily phase properties and the diameter of internal phase droplets; in diluted ME the small number of droplets had no important effect on viscosity, however when the number of droplets raise, droplet-droplet interactions become prevalent and the friction can lead to a raise in viscosity [14]. Another assumption is that in o/w systems with more than 50% of water, hydrophilic groups of surfactant are highly hydrated and interconnected by hydrogen bond, which lead to a gel structure [13]. The behavior seen in the ME presented herein, is possibly in accordance to a ME with sodium dodecyl sulfate and ethanol for which a viscous region was described, called region S, as a stable viscous region where phases L4 and Lα (vesicular and lamellar phase) coexist. That region S viewed by polarized microscopy showed large spherules dispersed in a continuous isotropic medium, being the weak birefringence due to the presence of such spherules. ME with low ethanol content are turbid and they get clear as ethanol content or temperature raise and the viscosity of the ME in this region is considerably affected by the preparation method [39]. In our study, images seen by phase contrast and polarized light microscopy would be consistent with this type of system. It is also important to consider that drug itself may alter phase behavior when it has surfactant property; however in this case AmB did not alter the gel-like ME despite of being amphiphilic.
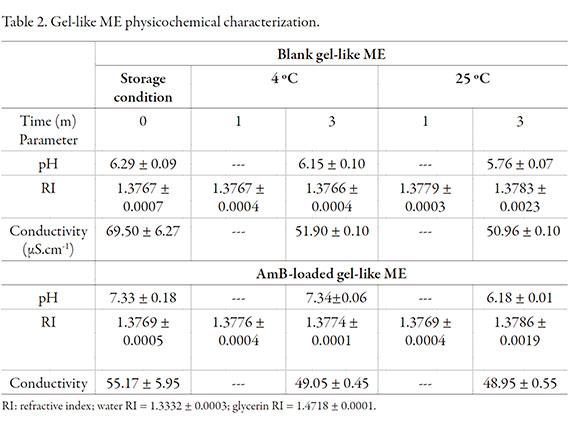
Physicochemical parameters are shown in Table 2. RI (refractive index) was no modified by the incorporation of AmB. Readings remained water < gel-like ME < glycerin during the period assayed. pH of blank sample was lower than the one loaded with AmB. The selected gel-like ME showed electroconductivity according to o/w ME, despite the non-ionic nature of main surfactant (parameter was assessed without the addition of electrolytes) and the viscosity of the sample. Conductivity was similar for the blank formulation than the loaded one (no significantly difference, p > 0.05), so we conclude the drug did not affect the external phase of the ME.
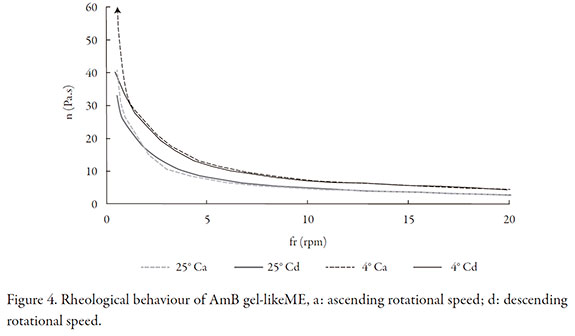
At the time of preparation the gel-like ME appears as a soft gel, both blank and the loaded one, that can be rotated in the beaker but samples showed a more rigid appearance after storage at 4 °C. AmB-loaded gel-like ME showed a pseudoplastic behavior without thixotropy (Figure 4). Samples stored at room temperature were visibly less viscous after three months and the system could easily flow through the vial wall. On basis of stability of AmB and gel structure, this formulation should be store at 4 °C.
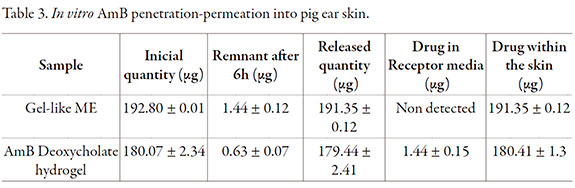
In vitro AmB penetration-permeation
Study showed that AmB penetrated almost completely within the skin (99.23 +/- 0.06%) without permeation to receptor fluid during the time of assay after application of AmB-loaded gel-like ME; the estimated rate of penetration into the skin was 10.16 +/- 0.01 µg/cm2/h. On the case of AmB deoxycholate hydrogel the drug penetrated the skin at the same extent (99.65 +/- 0.04%; penetration rate 9.56 +/- 0.05 µg/cm2/h), however drug was detected in receptor media after 1 h of application (Table 3). Permeation was statistically different for the two formulations (p < 0.05). At the end of assay drug was only detected in stratum cormeum (SC) for the AmB-loaded gel-like ME, whereas for the AmB deoxycholate hydrogel drug was found in SC, viable epidermis and dermis (data not shown) according to permeation behavior. AmB difussed completely into skin, however in the case of the AmB-loaded gel-like ME drug was retained within the skin during the assay, this longer residence time within the skin would help local effect and could prevent from systemic adverse effects.
As it was mentioned, ME have many advantages for topical administration of drugs, moreover o/w ME can behave as reservoir increasing local effect of drugs. In our study AmB diffusion within the skin was slower for the gel-like ME than the AmB deoxycholate hydrogel and the estimated penetration ratio into the skin was much higher than penetration results showed by other researchers when AmB was vehiculized in liposomes [28]. ME good skin penetration has already been study, nonetheless in this case the composition of the system could have helped even more. It was reported that some polyethylene ethers are effective skin penetration enhancers, because of their hydrophilic and lipophilic molecule portions they cause disruption of lipids in the SC and increase water content of the protein domain [8, 40].
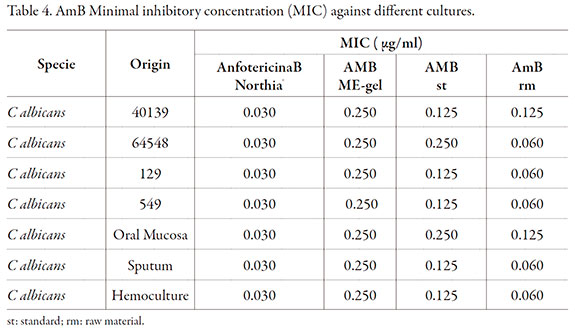
In vitro antifungal activity
In order to assess in vitro antifungal activity of AmB-loaded gel-like ME, MIC was determined for several strains of Candida and different patient isolates. Results are shown in Table 4. The MIC for AmB-loaded gel-like ME for Candida spp. was greater than MIC for AmB deoxycholate possibly due to viscosity of the formulation affecting drug diffusion in the culture media, since AmB deoxycholate was tested as solution. Nonetheless it has to be considered that our controls (AmB standard and AmB raw material) also had MIC values were greater than the reference for some samples, which could also explain the greater MIC results for the formulation assayed. Nevertheless we can conclude that AmB antifungal activity is appropriate for this novel formulation since susceptible species of fungi are inhibited by concentration of AmB ranging from 0.03 to 1.00 µg/ml but in vivo studies are necessary to assess clinical effectiveness [41].
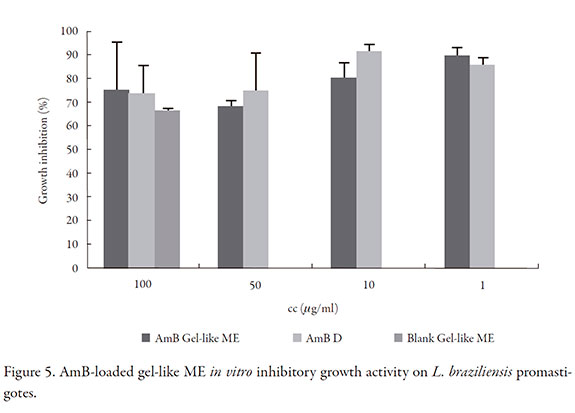
In vitro antileishmania activity
In vitro inhibitory activity of L. braziliensis promastigotes growth is shown in Figure 5. AmB loaded gel-like ME has the same activity as the reference AmB deoxycholate, for 100 µg/ml and 1 µg/ml concentration there was no significant difference (p > 0.05). However, blank formulation had inhibitory activity of parasite growth, 75.04 +/- 11.20% with no difference respect to AmB-loaded gel-like ME (p > 0.05); this fact was also reported for AmB solubilized with poloxamer 188 in a micellar formulation [42]. Furthermore, surfactants had strong lytic effect against Trypanosoma cruzi, a parasite of the same family as Leishmania, as was the case of Tween 20 and polyethylcyanoacrylate nanoparticles which had activity against parasite cells and enhanced activity of nifurtimox [43-45]. Surfactants modify parasite membrane, producing change in fluidity and leakage of vital substances, although in our study there was not synergism [46].
Modern therapies aim to deliver drugs in target sites with few adverse effects, this becomes a difficult task when dealing with intracellular parasites as is the case of Leishmania, and therefore the carrier for the drug takes a very important role.
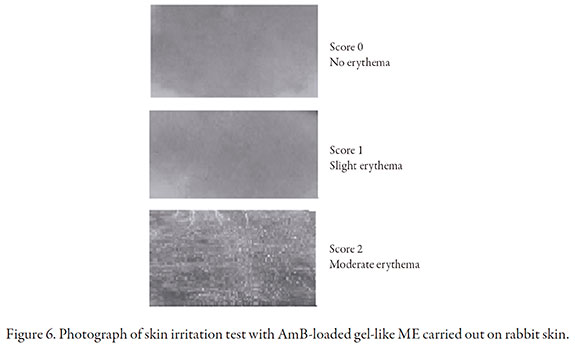
Skin irritation test
Cutaneous irritation of optimized formulation was tested using Draize test. Various degrees of irritation manifested by redness were observed showing a slight degree of irritation on rabbit skin although no oedema was observed (score: 2.08). The irritation responses were not homogenous across test animals. As shown in Figure 6 the maximum skin irritation was represented by moderate erythema, while some animals showed slight erythema. High surfactant concentrations and ethanol are known to be irritant, therefore the particular composition of this gel-like ME may be responsible for the observed effect on skin. Even considering this skin outcome, this novel formula has benefits concerning drug solubilization and pharmacological activity. An important fact to remark is that the drug did not reach the receptor compartment despite the formulation showing slight irritancy so AmB loaded gel-like ME could be safe for topical use.
Conclusion
A low cost gel-like ME of suitable viscosity for topical application was developed to deliver AmB. This novel formula was able to load a higher amount of AmB than drug solubility in water or several excipients and AmB remained stable for at least three months when stored in the fridge, as such, it could be used as a compounding formulation of AmB. Drug penetration into skin was significant so this AmB loaded gel-like ME is promising for the treatment of skin infections caused fungus and Leishmania parasites considering that there are few evidences of formulations that allow proper concentration of the drug within the skin after topical application. It would be of interest to further investigate this type of formulation by means of other techniques so as to provide details about its microstructure and also study its potential to be used as vehicle to solubilizing other drugs to be applied in the skin.
Acknowlegements
This work was financially supported with funds from Project UBACyT B003 from University of Buenos Aires. Authors thank PhD Rafael Ricco for his support in the obtention of phase-contrast images and Prof. PhD Emilio L. Malchiodi for his assistance with the antileishmanial in vitro assay.
References
1. M.J. Lawrence, G.D. Rees, Microemulsion-based media as novel drug delivery systems, Adv. Drug Deliv. Rev., 45, 89 (2000). [ Links ]
2. M. Kreilgaard, Influence of microemulsions on cutaneous drug delivery, Adv. Drug Deliv. Rev., 54, Suppl 1, 77 (2002). [ Links ]
3. S. Talegaonkar, A. Azeem, F.J. Ahmad, R.K. Khar, S.A. Pathan, Z.I. Khan, Microemulsions: A novel approach to enhanced drug delivery, Recent Pat. Drug Deliv. Formul., 2(3), 238 (2008). [ Links ]
4. B. Rozman, M. Gasperlin, E. Tinois-Tessoneaud, F. Pirot, F. Falson, Simultaneous absorption of vitamins C and E from topical microemulsions using reconstructed human epidermis as a skin model, Eur. J. Pharm. Biopharm., 72(1), 69 (2009). [ Links ]
5. L. Hua, P. Weisan, L. Jiayu, Z. Ying, Preparation, evaluation, and NMR characterization of vinpocetine microemulsion for transdermal delivery, Drug Dev. Ind. Pharm., 30(6), 657 (2004). [ Links ]
6. E. Peira, P. Scolari, M.R. Gasco, Transdermal permeation of apomorphine through hairless mouse skin from microemulsions, Int. J. Pharm., 226, 47 (2001). [ Links ]
7. Y.S. Rhee, J.G. Choi, E.S. Park, S.C. Chi, Transdermal delivery of ketoprofen using microemulsions, Int. J. Pharm., 228(1-2), 161 (2001). [ Links ]
8. E.-S. Park, S.-Y. Chang, M. Hahn, S.-C. Chi, Enhancing effect of polyoxyethylene alkyl ethers on the skin permeation of ibuprofen, Int. J. Pharm., 209, 109 (2000). [ Links ]
9. H.M. El-Laithy, K.M.F. El-Shaboury, The development of cutina lipogels and gel microemulsion for topical administration of fluconazole, AAPS PharmSciTech, 3(4), 35 (2002). [ Links ]
10. A. Azeem, Z.I. Khan, M. Aquil, F.J. Ahmad, R.K. Khar, S. Talegaonkar, Microemulsions as surrogate carrier for dermal drug delivery, Drug Dev. Ind. Pharm., 35, 525 (2009). [ Links ]
11. W.K. Kegel, T.G. Overbeek, H.N.W. Lekkerkerker, Thermodynamics of Microemulsions I. In: "Handbook of Microemulsion Science and Technology", P. Kumar, K.L. Mittal (editors), Marcel Dekker, New York, 1999, Chapter 2, pp. 13-44. [ Links ]
12. S.C. Sharma, K. Tsuchiya, K. Sakai, H. Sakai, M. Abe, R. Miyahara, A narrow bicontinuus microemulsion domain in mixed polymeric silicone systems, J. Oleo Sci., 57(12), 669 (2008). [ Links ]
13. F. Podlogar, M. Gasperlin, M. Tomsic, A. Jamnik, M. Bester Rogac, Structural characterization of water-Tween40®/Inwitor 308®- isopropyl myristate microemulsion using different experimental methods, Int. J. Pharm., 276, 115 (2004). [ Links ]
14. M.A. Correa, M.V. Scarpa, M.C. Franzini, A.G. Oliveira, On the incorporation of the non-steroidal anti-inflammatory naproxen into cationic O/W microemulsions. Colloids and Surfaces B, 43, 108 (2005). [ Links ]
15. T.P. Formariz, L.A. Chiavacci, V.H.V Sarmento, C.V. Santilli, E.S. Tabosa do Egito, A.G. Oliveira, Relationship between structural features and in vitro release of doxorubicin from biocompatible anionic microemulsion, Colloids and Surfaces B, 60, 28 (2007). [ Links ]
16. M.J. Lawrence, G.D Rees, Microemulsion-based media as novel drug delivery systems, Adv. Drug Deliv. Rev., 45, 89 (2000). [ Links ]
17. K. Trickett, J. Eastoe, Surfactant-based gels, Adv. Colloid Interface Sci., 144, 66 (2008). [ Links ]
18. M. Laupheimer, K. Jovic, F.E. Antunes, M. da G. Martins-Miguel, C. Stubenrauch, Studying orthogonal self-assembled systems: Phase behaviour and rheology of gelled microemulsions, Soft Matter, 9, 3661 (2013). [ Links ]
19. A. Lemke, A.F. Kiderlen, O. Kayser, Amphotericin B, Appl. Microbiol. Biotechnol., 68, 151 (2005). [ Links ]
20. N. Lincopani, E.M. Mamizukal, A.M. Carmona-Ribeiro, J. Antimicrob. Chemother., 52, 412 (2003). [ Links ]
21. R. Reithinger, J. Dujardin, C. Pirmez, B. Alexander, S. Brooker, Cutaneous leishmaniasis, Lancet Infect. Dis., 7(9), 581 (2007). [ Links ]
22. P. Minodiera, P. Parola, Cutaneous leishmaniasis treatment, Travel. Med. Infec. Dis., 5, 150 (2007). [ Links ]
23. K.M. Wasan, E.K. Wasan, P. Gershkovich, X. Zhu, R.R. Tidwell, K.A. Werbovetz, J.G. Clement, S.J. Thornton, Highly effective oral amphotericin B formulation against murine visceral leishmaniasis, J. Infec. Dis., 200, 357 (2009). [ Links ]
24. R.C. Zauli-Nascimento, D.C. Miguel, J.K.U. Yokoyama-Yasunaka, L.I.A. Pereira, M.A. Pelli de Oliveira, F. Ribeiro-Dias, M.L. Dorta, S.R.B. Uliana, In vitro sensitivity of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B, Trop. Med. Int. Health, 15, 68 (2010). [ Links ]
25. D. Vardy, Y. Barenholz, R. Cohen, A. Zvulunov, A. Biton, S. Klaus, S. Frankenburg, Topical amphotericin B for cutaneous leishmaniasis, Arch. Dermatol., 135, 856 (1999). [ Links ]
26. D. Vardy, Y. Barenholz, N. Naftoliev, S. Klaus, L. Gilead, S. Franknburg, Efficacious topical treatment for human cutaneous leishmaniasis, Trans. Roy. Soc. Trop. Med. Hyg., 95, 184 (2001). [ Links ]
27. A. Zvulunov, E. Cagnano, S. Frankenburg, Y. Barenholz, D. Vardy, Topical treatment of persistent cutaneous leishmaniasis with ethanolic lipid Amphotericin B, Pediatr. Infect. Dis. J., 22(6), 567 (2003). [ Links ]
28. A. Manosroi, L. Kongkaneramit, J. Manosroi, Stability and transdermal absorption of topical amphotericin B liposome formulations, Int. J. Pharm., 270(1-2), 279 (2004). [ Links ]
29. European Commission Scientific Committee on Consumer Products (SCCP) opinion on basic criteria for the in vitro assessment of dermal absorption of cosmetic ingredients, URL: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_s_03.pdf, March 2006. [ Links ]
30. European Commission Health & Consumer Protection Directorate General Sanco/222/2000 Rev. 7 19 Guidance Document on Dermal Absorption, URL: http://www.efsa.europa.eu/cs/BlobServer/DocumentSet/SANCO_222_2000_rev7.pdf?ssbinary=true, March 2004. [ Links ]
31. N. Hasler-Nguyen, D. Shelton, G. Ponard, M. Bader, M. Schaffrik, P. Mallefet, Evaluation of the in vitro skin permeation of antiviral drugs from penciclovir 1% cream and acyclovir 5% cream used to treat herpes simplex virus infection, BMC Dermatology, 9, 3 (2009). [ Links ]
32. National Committee for Clinical Laboratory Standards Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, Pa. 2002. [ Links ]
33. V.P. Sülsen, F.M. Frank, S.I. Cazorla, P. Barrera, B. Freixa, R. Vila, M.A. Sosa, E.L. Malchiodi, L.V. Muschieti, V.S. Martino, Psilostachyin C: A natural compound with trypanocidal activity, Int. J. Antimicrob. Agents, 37, 536 (2011). [ Links ]
34. J.H. Draize, G. Woodward, H.O. Calvery, Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes, J. Pharmacol. Exp. Ther., 8, 377 (1944). [ Links ]
35. D. Butani, C. Yewale, A. Misra, Amphotericin B topical microemulsion: Formulation, characterization and evaluation, Colloids and Surfaces B, 116, 351 (2014). [ Links ]
36. K.C. Pestana, T.P. Formariz , C.M. Franzini, V.H.V. Sarmento, L.A. Chiavacci, M.V. Scarpa, E.S.T. Egito, A.G. Oliveira, Oil-in-water lecithin-based microemulsions as potencial delivery system for amphotericin B, Colloids and Surfaces B, 66, 253 (2008). [ Links ]
37. C. Salerno, S. Pérez, E. Monteagudo, A. Carlucci, C. Bregni, Solubility of amphotericin B in water-lecithin-dispersions and lecithin-based submicron emulsions, Pak. J. Pharm. Sci., 26(1), 189 (2013). [ Links ]
38. E. Esposito, F. Bortolotti, E. Menegatti, R. Cortesi, Amphiphilic association systems for Amphotericin B delivery, Int. J. Pharm., 260, 249 (2003). [ Links ]
39. A.M. Belloc, Effects of alcohol chain length and salt on phase behavior and critical phenomena in SDS microemulsions. In: "Handbook of Microemulsion Science and Technology", P. Kumar, K.L. Mittal (editors), Marcel Dekker, New York, 1999, Chapter 6, pp. 139-184. [ Links ]
40. R.M. Hathout, T.J. Woodman, S. Mansour, N.D. Mortada, A.S. Geneidi, R.H. Guy, Microemulsion formulations for the transdermal delivery of testosterone, Eur. J. Pharm. Sci., 40(3), 188 (2010). [ Links ]
41. http://pubchem.ncbi.nim.gov/compound/5280965#section =Pharmacologyand-Biochemistry, October 2015. [ Links ]
42. S. Espuelas, P. Legrand, P.M. Loiseau, C. Bories, G. Barrat, J.M. Irache, In vitro reversion of amphotericin B resistance in Leishmania donovani by Poloxamer 188, Antimicrob. Agents Chemother., 44(8), 2190 (2000). [ Links ]
43. C.O. Rangel-Yagui, W.L. Hsu-Helen, L.R.S. Barbosa, C. Wilker, A. Pessoa, L.C. Tavares, R. Itri, Novel potential drug against T. cruzi and its interaction with surfactant micelles, Pharm. Dev. Tech., 12(2), 183 (2007). [ Links ]
44. G. Sánchez, D. Cuéllar, I. Zulantay, M. Gajardo, G.C. González-Martín, Cytotoxicity and trypanocidal activity of nifurtimox encapsulated in ethylcyanoacrylate nanoparticles, Biol. Res., 35(1), 39 (2002). [ Links ]
45. G. González-Martín, I. Merlino, M.N. Rodríguez-Cabezas, M. Torres, R. Núñez, A. Osuna, Characterization and trypanocidal activity of nifurtimox containing and empty nanoparticles of polyethylcyanoacrylates, J. Pharm. Pharmacol., 50(1), 29 (1998). [ Links ]
46. D. Dimitrijevic, A.J. Shaw, A.T. Florence, Effects of some non-ionic surfactants on transepithelial permeability in Caco-2 cells, J. Pharm. Pharmacol., 52(2), 157 (2000). [ Links ]














