Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418
Rev. colomb. cienc. quim. farm. vol.44 no.3 Bogotá Sept./Dec. 2015
Economic impact of vaccination with PCV13 vs. vaccination with PCV 10 in Colombia
Impacto económico de la vacunación con PCV13 frente al PCV 10 en Colombia
Jorge Díaz a
José Urrego b
Alexander Moreno c
Juan Reyes d
Fernando Peralta e
Víctor Prieto f
Paul Brown g
a Universidad Nacional de Colombia, Bogotá Campus, Faculty of Sciences, Department of Pharmacy. Carrera 30 No. 45-03, Bogotá, Colombia. E-mail: jadiazr@unal.edu.co.
b Universidad de Ciencias Aplicadas y Ambientales, Bogotá Campus, Faculty of Health Sciences. Calle 222 No. 55-37, Bogotá, Colombia. E-mail: joseurregon@gmail.com.
c Universidad Distrital Francisco José de Caldas, Faculty of Engineering. Carrera 8 No. 40-62, Bogotá, Colombia, ZIP Code 111321. E-mail: ingindmoreno@gmail.com.
d Pfizer. Avenida Suba 95-66, Bogotá, Colombia. E-mail: JuanManuel.Reyes@pfizer.com.
e Universidad Nacional de Colombia, Bogotá Campus, School of Medicine, Instituto de Investigaciones Clínicas. Carrera 30 No. 45-03, Bogotá, Colombia. E-mail: fperaltap@unal.edu.co.
f Pfizer, Avenida Suba 95-66, Bogotá, Colombia. E-mail: VictorAlfonso.Prieto@pfizer.com.
g University of California, Merced, Health Science Research Institute, 5200 North Lake Road, Merced, CA 95343, USA. E-mail: pbrown3@ucmerced.edu.
Recibido para evaluación: 21 de febrero de 2015.
Aceptado para publicación: 10 de diciembre de 2015.
Summary
We aim was to estimate the difference of costs and expected cases from serotype coverage of the 13-valent pneumococcal conjugated vaccine (PCV13) and 10-valent pneumococcal conjugated vaccine (PCV10) in the population under 5 years of age in Colombia, using a deterministic model. We considered the probabilities of incidence, mortality and sequelae from infections of pneumonia, meningitis, sepsis and acute otitis media, as well as the clinical effectiveness of PCV13 and PCV10, which were determined by a systematic review of the literature. A2 + 1 immunization schedule was considered, and a 42% herd effect and 84.09% population coverage were assumed. The perspective was the Colombian health system with a time horizon of 5-years. The model showed greater protection of PCV13 in comparison to PCV10. A difference of 98 prevented deaths was observed for meningitis, pneu monia and sepsis. The opportunity cost difference found in the 5-year follow-up between PCV13 and PCV10 vaccines was COP (Colombian pesos) 36,128,082,380 at 2012 prices, which represents COP 7,225,616,476 of difference per year. PCV13 is considered the better alternative, this is mainly due to the impact that this vaccine has on the disease burden of the infections produced by Streptococcus pneumoniae in Colombian children under five years of age.
Key words: Streptococcus pneumoniae, pneumococcal vaccines, health economics, Colombia.
Resumen
Nuestro objetivo fue estimar la diferencia de los costos y los casos que se esperan de la cobertura de serotipos de la vacunas conjugadas 13-valente neumocócica (PCV13) y 10-valente neumocócica (PCV10), en la población menor de cinco años de edad en Colombia, mediante un modelo determinista. Se consideraron las probabilidades de incidencia, mortalidad y secuelas de las infecciones de neumonía, meningitis, sepsis y la otitis media aguda, así como la efectividad clínica de la PCV13 y la PCV10, que se determinaron mediante una revisión sistemática de la literatura. Se consideró un esquema de vacunación A2 + 1 y se supuso un efecto grupal del 42% y una cobertura de la población de 84,09%. La perspectiva fue el sistema de salud colombiano, con un horizonte temporal de cinco años. El modelo mostró una mayor protección de PCV13 en comparación con PCV10. Se observó una diferencia de 98 muertes que se evitaron por causa de la meningitis, la neumonía y la sepsis. La diferencia costooportunidad encontrada en el seguimiento de cinco años entre las vacunas PCV13 y la PCV10 fue de 36,128,082,380 pesos colombianos (COP) a precios de 2012, lo que representa una diferencia de COP 7,225,616,476 por año.
Palabras clave: Streptococcus pneumoniae, vacunas neumocócicas, economía de la salud, Colombia.
Introduction
Streptococcus pneumoniae (Sp) or pneumococcus is one of the most frequent causes of clinical conditions such as pneumonia, meningitis, bacteremia, sepsis and acute otitis media (AOM) in children and adults. The pneumococcal disease is an important cause of morbidity, mortality and increased costs for the health system [1-4].
The World Health Organization (WHO) estimates that nearly 1.6 million persons, including 1,000,000 under-five children, die every year due to invasive pneumococcal disease (pneumonia, meningitis, sepsis) [3]. Thereby, every year are reported in the United States about 3,000 cases of meningitis, 50,000 cases of bacteremia, 500,000 cases of pneumonia and 7,000,000 of AOM [5, 6].
In developing countries the invasive pneumococcal disease is one determining factor of infant mortality. The case-fatality rate of pneumococcal pneumonia is 10-20%. In patients with invasive pneumococcal disease this rate may be as high as 40% [2].
A cost-effectiveness study conducted in Colombia, which was based on a hypothetical cohort of neonates born in 2009, showed that in absence of vaccination against pneumococcus there may be produced 701,326 to 812,796 cases of AOM, 14,670 to 24,020 cases of ambulatory and hospitalized radiological pneumonia and 380 to 633 cases of pneumococcal meningitis [7, 8].
The Ministry of Social Protection estimated that in Colombia from 8,000 to 10,000 cases per year of pneumococcal invasive disease and around 700 deaths of children less than 2 years of age occurred in 2006 [9].
As the pneumococcal disease is a public health priority in Colombia because it represents not only a high disease burden but also an economic impact on the care costs of above diseases and patient's productivity loss, the objective of this study was to estimate the difference of costs and expected cases from the serotype coverage of the 13-valentand 10-valent pneumococcal conjugated vaccines (PCV13and PCV10, respectively) in the population under five years in Colombia.
Methods
A deterministic model with a fixed cohort of newborn children in Colombia was built. Information provided in 2011by the National Department of Statistics (DANE) was used [10]. A 5-year simulation which included probabilities of incidence, mortality, pneumonia sequelae, meningitis, sepsis and AOM, as well as the clinical effectiveness of the conjugate vaccines PCV13 and PCV10 was performed.
The 2 + 1 vaccination schedule established in Colombia was used; in this schedule are administered doses at 2 and 4 months of age with a boosterat1-year of age. A 42% herd effect was assumed based on the Poehling et al. work [11] who studied the PCV7 effect on the protection rate in the non-vaccinated population (children between 0 and 90 days of age) which were living with vaccinated children. The vaccination coverage used for the model was 84.09% in accordance with information reported by the Ministry of Health and Social Protection [12]. For the study vaccines the differences in protection were expressed as opportunity costs and net benefit.
Determination of the probabilities included in the model
The probabilities of incidence, mortality and sequelae of the study diseases in the population of children fewer than five years of age were estimated from a systemic review. The databases used for the search were: Medline, The Cochrane Library, BIREME, Science Direct and Google Scholar. Two independent investigators evaluated for inclusion the references obtained from the search, and the disagreements were resolved by consensus.
A search filter for population under five years of age was used, and for determining the mortality and incidence of diseases caused by S. pneumoniae, the MESH terms Streptococcus pneumoniae, Pneumonia, Otitis media, Bacteremia, Meningitis, Incidence, Mortality, Disease burden and epidemiology were used.
For an improved sensibility in the search of the sequelae of the diseases included, the following free text terms were used: Pneumococcus, Acute Otitis Media (AOM), Meningitis, Bacteremia, Pneumonia, sequelae and complications. In addition, in order to establish the sequelae mostly affecting patients with history of meningitis and AOM a consultation to experts was made. Sequelae from pneumonia and sepsis were not considered.
Determination of the conjugate vaccines effectiveness
A systematic search of randomized controlled clinical studies evaluating effectiveness of PCV10 and PCV13 was conducted. When clinical studies with the two vaccines were not available, studies comparing the vaccine with placebo were used. For cases where no information was found, the adjusted effectiveness of PCV10 and PCV13 was estimated, under the assumption that the effectiveness will increase proportionally with the number of covered serotypes, and the PCV7 clinical effectiveness was used as reference. The PCV13 and PCV10 effectiveness for prevention of sepsis and meningitis was calculated by multiplying the PCV7 effectiveness by the proportion of serotypes covered of each vaccine in the local isolations reported; this method already has been previously proposed [13-15].
The clinical effectiveness values of PCV7 for AOM, pneumonia, sepsis and meningitis were obtained. The information about effectiveness corresponded to the prevention of diseases above described, where the only causative agent was S. pneumoniae. The distribution of serotypes was taken from SIREVA II reports which are specific to isolations in the country for the years 2009-2011 in children under 59 months of age [16].
Costs of the health interventions
Direct costs of consumption of medical resources for interventions were determined in the study population using costs information provided by a Healthcare Promoting Entity (HCPE) providing healthcare services nationwide, which included direct medical costs for healthcare services and cases-type from the information taken of healthcare guides and consultation to clinical experts were created. The identifying of cost-generating events was carried out by a bottom up methodology. Once identified the different procedures and validated by consultation to clinical experts, the cost values were estimated in accordance with the ISS 2001 + 25% and 2013 SOAT tariffs manuals applicable in Colombia.
The perspective of the health system was applied in the costing process, which uses as parameters of quantification of cost-generating events, manuals recognized as economic parameters for recognition of services within contract models.
Indirect costs were related to the mortality and sequelae produced by the studied diseases. The mortality was estimated as years of potential life lost (YPLL), which was estimated according to the adapted YPLL ipc (invester-producer-consumer) model of Gardner and Sanborn [17]. This model weighs the deaths based on a balance between investment, production and potential consumption by each person in accordance with the productivity stage in which the person dies. This method divides the lifetime of a person into three periods: investment period (0 to 11 y), production period (12 to 62 y) and consumption period (62 y +), adjusted for Colombia under the Pension Amendment Act 797 of January 29, 2003 [18]. On the other hand, the sequelae were measured in life-years saved (LYS) which consist of the life-years lived with disability added to life-years lost because of premature mortality [19].
Time horizon
The health outcomes calculated for the 5-year period were discounted at a 3% annual rate, and the costs were not discounted given that they not were calculated for the future. Current (mean) values of 2012 were used, which then were multiplied with each one of the health outcomes (prevented disease cases, deaths and sequelae).
Results
Data used in the model are summarized in Table 1. The cohort of children born in Colombia over the year 2011 was 652,616 children.
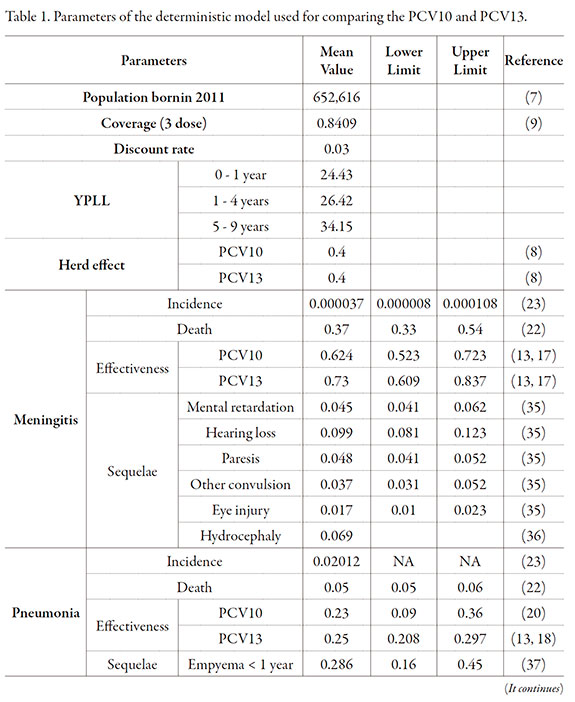
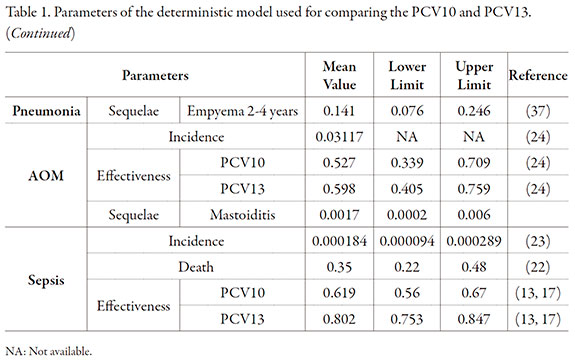
To modeling the PCV10 and PCV13 effectiveness, taking as a basis the PCV7 results, was used the Northern California Kaiser Permanent (NCKP) study [20-22]. NCKP was a randomized double-blind clinical study conducted in 37,868 children under 15 months of age who were vaccinated with PCV7. The incidence of invasive pneumococcal disease, acute otitis media and pneumonia was evaluated. The reported effectiveness for invasive pneumococcal disease prevention by PCV7 for each serotype included in the vaccine was 97.4% (95% CI: 82.7%, 99.9%). For the pneumonia prevention effectiveness of PCV13, the PCV7 prevention effectiveness of 25.5% (95% CI: 6.5%, 40.7%) was used in accordance with Hansen et al. study [21].
A study evaluating the pneumonia prevention effectiveness of PCV10 in Latin American patients by Tregnaghi et al. was identified [23]; they estimated that the vaccine efficacy was 23% (95% CI: 9%, 36%) (Table 1).
For estimation of the prevention effectiveness of pneumococcal AOM per vaccine serotype by PCV13, the PCV7 effectiveness reported in Black et al. study [20] was used, which was 64.7% (p = 0.035), with an 83% distribution of the seven serotypes covered by the study. An adjustment was made in accordance with the distribution of vaccine serotypes in a Colombian population reported by Sierra et al. [24]. Finally, for the effectiveness of PCV10 for prevention of the pneumococcal AOM per vaccine serotypes was used as reference the 11-serotype conjugate vaccine presented in the Prymula et al. study [25], which was 52.6% (95% CI: 35.0%, 65.5%). The distribution of serotypes covered by the vaccine in the study was 65.93%. The effectiveness adjustment was made in accordance with the distribution of serotypes reported by Sierra et al. [24].
Direct costs are reported in Table 2, with the method used for their calculation. The model results showed that PCV13 prevented 12 meningitis cases more than the PCV10. In addition, 1,187; 11,353 and 100 cases may be prevented of pneumonia, acute otitis media and sepsis, respectively (Table 3). The total number of deaths prevented was 98, which were mostly caused by pneumonia and sepsis, and corresponds to 2,714 YPLL.
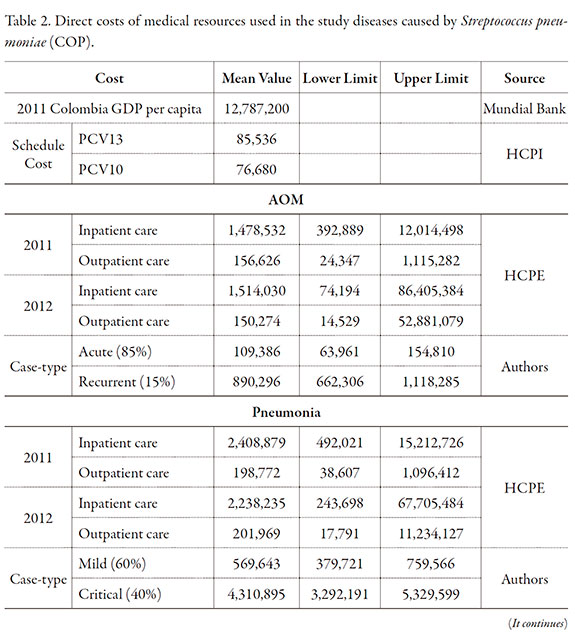
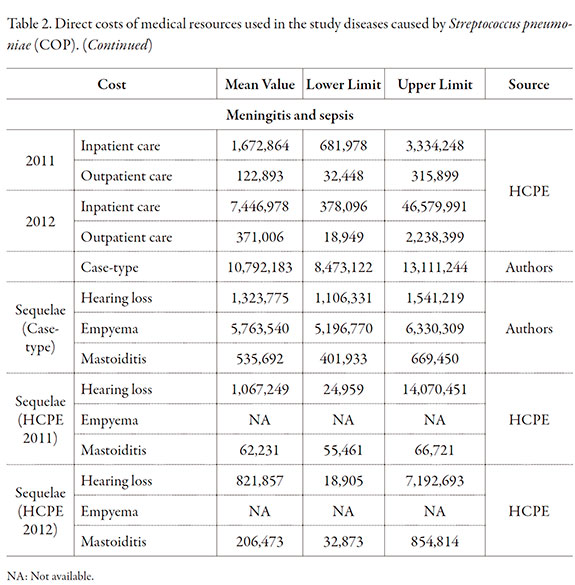


No differences were found in the sequelae secondary to meningitis (Table 3). However, 68 and 100 empyema cases were prevented in infants under one year of age and in children 2-4 years old, respectively. Nineteen cases of mastoiditis sequelae were estimated to have been prevented by the PCV13.
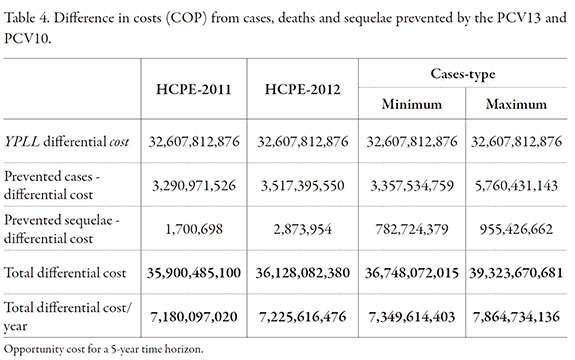
The health outcomes not covered by the PCV10 use, expressed in Colombian currency (pesos) at 2012 prices (opportunity cost) ranged between a minimum of COP 36,748,072,015 and a maximum of COP 39,323,670,681, with an annual average between COP 7,349,614,403 and 7,864,734,136. The 82.9% represents the productivity loss associated with early deaths, 14.6% with morbidity cases and, in a lower percentage, sequelae owing to their low incidence (Table 4).
When the health outcomes were evaluated as the prevented cases and bearing in mind only the vaccines costs, if the modeled cohort were vaccinated with PCV13, a discounted net benefit ranging between COP 300,705,179,186 and COP 325,848,596,224 would be obtained; and if the same population were vaccinated with PCV10 the discounted net benefit would range between COP 263,957,107,171 and COP 286,524,925,543.
This study shows the opportunity costs achieved with the PCV13 in children under 5 years of age in comparison to the PCV10, which generates savings for the Colombian health system if the PCV13 were used.
Basically, the opportunity cost was determined in this occasion by the number of prevented deaths. The lower number of deaths resulting from pneumococcus disease in the 2011 cohort of children vaccinated with PCV13 contributed to achieve greater opportunity costs. According to the results, a greater death proportion by sepsis and pneumonia could be prevented.
From the epidemiologic standpoint, the evidence available on the disease burden by Streptococcus pneumoniae in Colombia is very low, owing to the lower number of studies in the country. Valenzuela et al. [26] demonstrated that no studies on this topic had been conducted in Colombian before 2006.
According to the literature review conducted, the only study assessing the Streptococcus pneumoniae behavior in Colombian children under five years of age was performed by Benavides et al. [27] who studied the frequency of the invasive pneumococcal disease and pneumonia. However, it should be borne in mind the risk of a biased selection if considered that the study was exclusively performed on contributive health system population.
Taking the data published by Benavides et al. [27] and the Valenzuela et al. [26] results, it is possible to compare the frequency of the different diseases in Colombia and Latin America, respectively. The meningitis incidence in the country was lower than in the rest of Latin America, the meningitis incidence in children under 5 years of age reported by Valenzuela was 11 per 100,000-years.
For pneumonia, the behavior is similar between the national incidence and that of Latin America which was 2.834 cases per 100,000-years. On the other hand, the sepsis incidence used in the present document reported in the Benavides et al. study [27] was greater than that reported by Valenzuela et al. [26] of 12 cases per 100,000-years.
It is important to highlight that for OMA the effectiveness against serotypes included in the vaccine is considered based on the indications approved both to PCV10 and PCV13.
For children under five years in Colombia are available results from the Sierra et al. study [24] on the etiological distribution of children diagnosed with AOM. The results from this study showed that Streptococcus pneumoniae causes the 30% otitis media cases. Above data are consistent with the information furnished by Bardach et al. [28] who found a 32.4% (95% CI: 27.1%, 38.0%) incidence of otitis media for Latin America and Caribe.
In this study the SIREVA II program results [16] were used to determine the proportion of cases ascribable to each serotype, in accordance with the Streptococcus pneumoniae isolations in the invasive pneumococcal disease cases between 2009 and 2011. Seventy per cent (70%) of the isolations corresponded to the serotypes 14, 1, 6b, 19a, 3,18c and 23f.
In the calculation of PCV10 effectiveness the protective effect of the protein D against non-typeable H. influenza was not considered by us. Studies have shown that the effectiveness against non-typeable H. influenzae of this vaccine is controversial as evidenced by COMPAS [29] and Van den Bergh et al. [30] studies, in which no significant differences of prevention of otitis media and nasopharyngeal colonization were found from the clinical viewpoint.
Furthermore, the PCV10 cross protection to the serotype 19a was not considered, due to the protection against the 19f group. On the other hand, the PCV10 shows no cross protection against 19a and 6a, according to the COMPAS study [29]. There is evidence that the PCV7 vaccine which also protects against the serotype 19f shows no protection against serotype 19a as proven in a study conducted in Canada where its effectiveness was lower than 42% (95% CI: -76 -79%) [31]. In another study conducted in the United States, an increase of the serotype 19a was observed from the inclusion of PCV7 in the immunization program, a condition which evidences absence of protection against this serotype [32].
The PCV13 effectiveness against serotype 3 has been under discussion. Studies, such as that of Miller et al. in 2011 [33] have proven this ineffectiveness. However, this study involves the problem of having very small sample size that lead, therefore, to somewhat inaccurate results. This is also discussed by the Joint Committee on Vaccination and Immunization which concluded that few were the invasive disease cases produced by the serotype 3 in the place where the study was performed [34]. However, in the United States, the results of a study performed in 8 pediatric care centers showed that after implemented the use of PCV13, a 68% reduction of serotype 3 was observed in a 1-year period [35].
Owing to resources shortage, trade-offs are often necessary in medical decision taking. Gafni and Birch [36] make emphasis on the threshold value in the cost-effectiveness studies for decision making. However, decision makers need information on opportunity costs of a political decision (i.e., the next-best alternative which should be aban doned as a result of the decision) for an efficiently improved allocation of resources [36]. Opportunity costs are health outcomes which might be achieved with other interventions not performed if these resources were compromised to the chosen intervention [37]. In the international context there are very few studies using the concept of opportunity cost to evaluate the impact of the use of vaccines and there are not publications related to pneumococcal vaccine.
An important advantage of our study is that an attempt was made of working with data originated in the country. Data recently published by Benavides were not available when Alvis and De la Hoz [38] and Castañeda et al. [15] performed their economic studies. In these studies data from Latin American countries were used as reference. In the same way the herd effect and sequelae effect expressed as LYS, have been taken into account.
The costs of the different interventions were obtained from local information supplied by a Health Promotion Entity (HCPE), which provide information under actual conditions within the market.
In addition, the costs estimated from the provided information (HCPE) and the information obtained from the construction of cases-type, corroborates that the costs reported by HCPE are within the ranges estimated for costs based on the intervals proposed within the cases-type.
It should be borne in mind that since HCPE information is based on contract models established with the different Health Care Providing Institutions (HCPIs), the cost differences with the cases-type are determined by this type of characteristics in the Colombian health system.
Among the limitations of this study was the unavailability of studies conducted in Colombia allowing to elucidate the actual epidemiological situation generated by Streptococcus pneumonia in the study population, the unavailability of disease cost studies, the assumed adjustment of the effectiveness of the vaccines evaluated due to deficiency of the randomized clinical studies, and the unavailability of accurate diagnosis of patients with Streptococcus pneumoniae in the cases of AOM and pneumonia because the patients' diagnosis is usually based on radiological and clinical criteria which often lead to problems in the actual estimation of the disease caused by an infectious agent.
Conclusions
It was found that with the alternative PCV13 there would be less AOM, sepsis and pneumonia cases owing to additional coverage by this vaccine of the serotypes 3, 6a and 19a which correspond to 17.32% of the Streptococcus pneumonia distribution in children under 5 years of age between 2009 and 2011.For meningitis these three serotypes constitute 10.94% and for sepsis and pneumonia 18.71% of the total serotypes isolated in Colombia.
If the vaccination is performed with the PCV13 alternative, the deaths in the 2011 children cohort associated with pneumococcal disease would be lower.
The health outcomes not covered by the use of vaccine PCV10, expressed in Colombian currency (pesos) at the 2012 prices (opportunity cost), range between a minimum and maximum amount of COP 36,748,072,015 and COP 39,323,670,681, respectively, with an annual mean between 7,349,614,403 and 7,864,734,136, where 82.9% of this value represents the productivity loss associate with early deaths, 14.6% the morbidity cases and in a lower percentage by the sequelae due to their low incidence.
When all the health outcomes are evaluated as prevented cases and take into account only the vaccines costs, the immunization with PCV13 of the modeled cohort would be obtained a discounted net benefit ranging between COP 300,705,179,186 and COP 325,848,596,224, and if the same population were vaccinated with PCV10, the net benefit would range between COP 263,957,107,171 and COP 286,524,925,543.
Competing interests
The economic evaluation was funded by Laboratorios Pfizer S.A.S.
References
1. World Health Organization, Report of a meeting on priorities for pneumococcal and Haemophilusin.uenzae type b (Hib) vaccine development and introduction, URL: http://whqlibdoc.who.int/hq/2001/WHO_V&B_01.14.pdf, February 2013. [ Links ]
2. World Health Organization, Pneumococcal vaccines, Wkly Epidemiol. Rec., 78, 110 (2003). [ Links ]
3. World Health Organization, Pneumococcal conjugate vaccine for childhood immunization -WHO position paper, Wkly Epidemiol. Rec., 82, 93 (2007). [ Links ]
4. K. Edmond, A. Clark, V.S. Korczak, C. Sanderson, U.K. Griffiths, I. Rudan, Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis, Lancet Infect. Dis., 10, 317 (2010). [ Links ]
5. J. Casado, J. Aristegui, C.R. de Liria, J.M. Martinon, C. Fernández, Clinical data and factors associated with poor outcome in pneumococcal meningitis, Eur. J. Pediatr., 165, 258 (2006). [ Links ]
6. CDC, Recommendations of the Immunization Practices Advisory Committee (ACIP). Update Pneumococcal polysaccharide vaccine usage -United States. Morb. Mortal. Wkly. Rep., 33, 273 (1984). [ Links ]
7. P. Brotons, G. Gelabert, C. Launes, E. Sicuri, R. Pallares, C. Munoz, Cost of hospitalizing children with invasive pneumococcal pneumonia, Vaccine, 4, 1117 (2013). [ Links ]
8. C. Castañeda, N. Alvis, A.J. Paternina, F. de la Hoz-Restrepo, Cost-effectiveness of the introduction of the pneumococcal polysaccharide vaccine in elderly Colombian population, Vaccine, 29, 7644 (2011). [ Links ]
9. C. Castañeda, N. Alvis, F. de la Hoz. The impact of pneumococcal disease on adults living in Bogotá, Colombia, Rev. Salud Pública, 12, 38 (2010). [ Links ]
10. Nacimientos 2011, Departamento Administrativo Nacional de Estadística (DANE), URL: http://www.dane.gov.co/index.php?option=com_content&view=article&id=1295&Itemid=119, February 2013. [ Links ]
11. K.A. Poehling, T.R. Talbot, M.R. Griffin, A.S. Craig, C.G. Whitney, E. Zell, C.A. Lexau, A.R. Thomas, L.H. Harrison, A.L. Reingold, J.L. Hadler, M.M. Farley, B.J. Anderson, W. Schaffner, Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine, JAMA, 259, 1668 (2006). [ Links ]
12. Ministerio de Salud y Protección Social, Sistema Integral de Información de la Protección Social, URL: http://www.sispro.gov.co/Pages/Contruya%20Su%20Consulta/Vacunacion.aspx, February 2013. [ Links ]
13. M.H. Rozenbaum, E.A. Sanders, A.J. van Hoek, A.G. Jansen, A. van der Ende, G. van den Dobbelsteen, G.D. Rodenburg, E. Hak, M.J. Postma, Cost effectiveness of pneumococcal vaccination among Dutch infants: Economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines, BMJ, 340, c2509 (2010). [ Links ]
14. J. Díez, M. Ridao, M.V. Gutiérrez, J. Puig, J.A. Lluch, E. Pastor, Pharmacoeconomic assessment of implementing a universal PCV-13 vaccination programme in the Valencian public health system (Spain), Vaccine, 29, 9640 (2011). [ Links ]
15. C. Castañeda, N. Alvis, M. Velandia, F. de la Hoz, Cost-effectiveness of pneumococcal conjugate vaccines of 7, 10, and 13 valences in Colombian children, Vaccine, 30, 1936 (2012). [ Links ]
16. Organización Panamericana de la Salud, SIREVA II (Sistema de Redes de Vigilancia de los Agentes Responsables de Neumonías y Meningitis Bacterianas), Washington, USA: Organización Panamericana de la Salud, URL: http://new.paho.org/hq/index.php?option=com_content&view=category&layout=blog&id=3609&Itemid=3953&lang=fr, February 2013. [ Links ]
17. J.W. Gardner, J.S. Sanborn, Years of potential life lost (YPLL) -What does it measure?, Epidemiology, 1, 322 (1990). [ Links ]
18. Ley 797 de 2003. Por la cual se reforman algunas disposiciones del sistema general de pensiones previsto en la Ley 100 de 1993 y se adoptan disposiciones sobre los regímenes pensionales exceptuados y especiales (2003), URL: http://www.alcaldiabogota.gov.co/sisjur/normas/Norma1.jsp?i=7223, February 2013. [ Links ]
19. C. Mathers, T. Vos, A. López, J. Salomón, M. Ezzati, National burden of disease studies: A practical guide. Edition 2.0. Global Program on Evidence for Health Policy. Geneva: World Health Organization, URL: http://www.who.int/healthinfo/nationalburdenofdiseasemanual.pdf, February 2013. [ Links ]
20. S. Black, H. Shinefield, B. Fireman, E. Lewis, P. Ray, J.R. Hansen, L. Elvin, K.M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group, Pediatr. Infect. Dis. J., 19, 187 (2000). [ Links ]
21. J. Hansen, S. Black, H. Shinefield, T. Cherian, J. Benson, B. Fireman, E. Lewis, P. Ray, J. Lee, Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: Updated analysis using World Health Organization standardized interpretation of chest radiographs, Pediatr. Infect. Dis. J., 25, 779 (2006). [ Links ]
22. S.B. Black, H.R. Shinefield, S. Ling, J. Hansen, B. Fireman, D. Spring, J. Noyes, E. Lewis, P. Ray, J. Lee, J. Hackell, Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia, Pediatr. Infect. Dis. J., 21, 810 (2002). [ Links ]
23. M.W. Tregnaghi, X. Sáez, P. López, H. Abate, E. Smith, Evaluating the efficacy of 10 valent pneumococcal non-typeable Haemophilus Influenzae protein-D conjugate vaccine (PHID-CV) against community-acquired pneumonia in Latin America, Abstract 412, Congreso SLIPE, 2011. [ Links ]
24. A. Sierra, P. López, M.A. Zapata, B. Vanegas, M. Castrejon, R. Deantonio, W.P. Hausdorff, R.E. Colindres, Nontypeable Haemophilus influenzae and Streptococcus pneumoniae as primary causes of acute otitis media in Colombian children: A prospective study, BMC Infect. Dis., 11, 4 (2011). [ Links ]
25. R. Prymula, P. Peeters, V. Chrobok, P. Kriz, E. Novakova, E. Kaliskova, I. Kohl, P. Lommel, J. Poolman, J.P. Prieels, L. Schuerman, Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: A randomised double-blind efficacy study, Lancet, 367, 740 (2006). [ Links ]
26. M.T. Valenzuela, R. O'Loughlin, F. de la Hoz, E. Gómez, D. Constenla, A. Sinha, J.E. Valencia, B. Flannery, C.A. de Quadros, The burden of pneumococcal disease among Latin American and Caribbean children: Review of the evidence, Rev. Panam. Salud Pública, 270, 25 (2009). [ Links ]
27. J.A. Benavides, O.O. Ovalle, G.R. Salvador, S. Gray, D. Isaacman, G.L. Rodgers, Population-based surveillance for invasive pneumococcal disease and pneumonia in infants and young children in Bogotá, Colombia, Vaccine, 30, 5886 (2012). [ Links ]
28. A. Bardach, A. Ciapponi, S. García, D. Glujovsky, A. Mazzoni, A. Fayad, R.E. Colindres, A. Gentile, Epidemiology of acute otitis media in children of Latin America and the Caribbean: A systematic review and meta-analysis; Int. J. Pediatr. Otorhinolaryngol., 75, 1062 (2011). [ Links ]
29. COMPAS: A phase III study to demonstrate efficacy of GlaxoSmithKline Biologicals' 10-valent pneumococcal vaccine (GSK1024850A) against Community Acquired Pneumonia and Acute Otitis Media GSK1024850A - Synflorix ™ (10Pn): GlaxoSmithKline (GSK) Biologicals' 10-valent pneumococcal conjugate vaccine 2012, URL: http://download.gsk-clinicalstudyregister.com/files/42228168-2da4-4fef-8442-299505cfe6ec, February 2013. [ Links ]
30. M.R. van den Bergh, J. Spijkerman, K.M. Swinnen, N.A. Francois, T.G. Pascal, D. Borys, L. Schuerman, E.P. Ijzerman, J.P. Bruin, A. van der Ende, R.H. Veenhoven, E.A. Sanders, Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: A randomized controlled trial, Clin. Infect. Dis., 56, 30 (2013). [ Links ]
31. G. Deceuninck, P. de Wals, N. Boulianne, G. de Serres, Effectiveness of pneumococcal conjugate vaccine using a 2 + 1 infant schedule in Quebec, Canada Pediatr. Infect. Dis. J., 29, 546 (2010). [ Links ]
32. M.R. Moore, R.E. Gertz, R.L. Woodbury, G.A. Barkocy-Gallagher, W. Schaffner, C. Lexau, K. Gershman, A. Reingold, M. Farley, L.H. Harrison, J.L. Hadler, N.M. Bennett, A.R. Thomas, L. McGee, T. Pilishvili, A.B. Brueggemann, C.G. Whitney, J.H. Jorgensen, B. Beall, Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005, J. Infect. Dis., 197, 1016 (2008). [ Links ]
33. E. Miller, N.J. Andrews, P.A. Waight, M.P. Slack, R.C. George, Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine, Vaccine, 29, 9127 (2011). [ Links ]
34. Joint Committee on Vaccination and Immunization. Pneumococcal Subcommittee, URL: http://media.dh.gov.uk/network/261/files/2012/07/JCVI-minutes-Pneumococcal-sub-committee-meeting-held-on-30-May-2012.pdf, July 2013. [ Links ]
35. S.L. Kaplan, W.J. Barson, P.L. Lin, J.R. Romero, J.S. Bradley, T.Q. Tan, J.A. Hoffman, L.B. Givner, E.O. Mason, Early trends for invasive Pneumococcal infections in children after the introduction of the 13-valent Pneumococcal conjugate vaccine, Pediatr. Infect. Dis. J., 32, 203 (2013). [ Links ]
36. A. Gafni, S. Birch, Incremental cost-effectiveness ratios (ICERs): The silence of the lambda, Soc. Sci. Med., 62, 2091 (2006). [ Links ]
37. M.F. Drummond, B. O'Brien, G.L. Stoddart, G.W. Torrance, "Methods for the economic evaluation of health care programmes", Oxford University Press, New York, 1997. [ Links ]
38. N. Alvis, F. de la Hoz, Cost effectiveness of heptavalent pneumococcal conjugate vaccine in populations of high risk in Colombia, Colomb. Med., 41, 315 (2010). [ Links ]














