Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418
Rev. colomb. cienc. quim. farm. vol.45 no.2 Bogotá May/Aug. 2016
https://doi.org/10.15446/rcciquifa.v45n2.59936
http://dx.doi.org/10.15446/rcciquifa.v45n2.59936
Synthesis, characterization and antibacterial screening of some Schiff bases derived from pyrazole and 4-amino antipyrine
Síntesis, caracterización y evaluación antibacteriana de algunas bases de Schiff derivadas de pirazol y 4-amino antipirina
Shipra Baluja1, Sumitra Chanda2
1 Department of Chemistry, Saurashtra University, Rajkot-360 005, Gujarat, India E-mail: shipra_baluja@rediffmail.com
2Department of Biosciences, Saurashtra University, Rajkot-360 005, Gujarat, India
Received: September 22, 2015 Accepted: April 7, 2016
Summary
Some Schiff bases of pyrazole and 4-amino antipyrine have been synthesized. The antibacterial screening of these synthesized compounds was done in dimethyl forma-mide against four Gram positive bacteria viz. Bacillus cereus, Staphylococcus aureus, Staphylococcus epidermidids and Micrococcus luteus, and three Gram negative bacteria viz. Proteus mirabilis, Escherichia coli and Klebsiella aerogenes. It is observed that in comparison to Schiff bases of 4-amino antipyrine, pyrazole Schiff bases are better for inhibition for these selected Gram positive and Gram negative bacterial strains.
Keywords: Pyrazole, 4-amino antipyrine, Schiff bases, dimethyl formamide, antibacterial activity.
Resumen
Se sintetizaron algunas bases de Schiff a partir de pirazol y 4-amino antipirina. La evaluación de la actividad antibacteriana de estos compuestos en dimetil formamida se realizó frente a cuatro bacterias Gram positivas, Bacillus cereus, Staphylococcus aureus, Staphylococcus epidermidids y Micrococcus luteus, y frente a tres bacterias Gram negativas, Proteus mirabilis, Escherichia coli y Klebsiella aerogenes. Se observó mejor inhibición bacteriana frente a las diferentes cepas para las bases de Schiff basadas en pirazol comparadas con aquellas basadas en 4-amino antipirina.
Palabras clave: Pirazol, 4-amino antipirina, bases de Schiff, dimetil formamida, actividad antibacteriana.
Introduction
Schiff 's bases are an important class of organic compounds having a wide variety of applications in many fields such as analytical, biological and inorganic chemistry [1-5]. Some of these compounds act as corrosion inhibitors [6] and are used as catalysts in polymer [7-8] and dyes [9] industries. Further, Schiff bases have gained importance in medicinal and pharmaceutical fields due to a broad spectrum of biological activities like antimicrobial [10-11], antifungal [12-13], antiviral [14], anti-inflammatory [15-16], analgesic [17-18], anticonvulsant [19], antitubercular [20], anticancer [21], antioxidant [22-23], anthelmintic [24], antimalarial [25-26] and so forth.
In continuation of our previous research [27-28], in the present work, some new Schiff bases have been synthesized having pyrazole and 4-amino antipyrine moieties and their structure were confirmed by IR, NMR and mass spectral data. The screening of these compounds was also done to study their antibacterial properties in dimethyl formamide.
Experimental
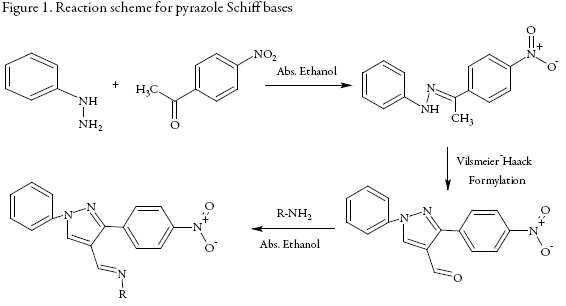
Synthesis of pyrazole Schiff bases
Synthesis of (1E)-1-(4-nitrophenyl)ethanone phenylhydrazone
Equimolar solution of phenyl hydazine and p-nitro phenyl acetophenone in absolute ethanol was refluxed in water bath for 2 hours using glacial acetic acid as catalysis. The crude product was isolated and was crystallized from absolute alcohol.
Synthesis of 3-(4-nitrophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde
(1E)-1-(4-nitro phenyl) ethanone phenyl hydrazone (0.01M) was added to Vilsmayer-Haack reagent (prepared by drop wise addition of 3 ml POCl3 in ice cooled 25 ml DMF) and was refluxed for 5 hours. The reaction mixture was poured into ice followed by neutralization using sodium bicarbonate. The crude product was isolated and crystallized from ethanol.
Synthesis of Schiff bases
In an ethanolic solution of 3-(4-nitro phenyl)-5-phenyl-4H-pyrazole-4-carbaldehyde and different aromatic amines, 2-3 drops of glacial acetic acid was added and the reaction mixture was refluxed for 10 hours. The resulting solution was cooled to room temperature and was poured in crushed ice with constant stirring. The product was filtered and washed with sodium bisulfate solution to remove the unreacted aldehyde. The crude product was crystallized from methanol and dried.
The synthesized Schiff bases are:
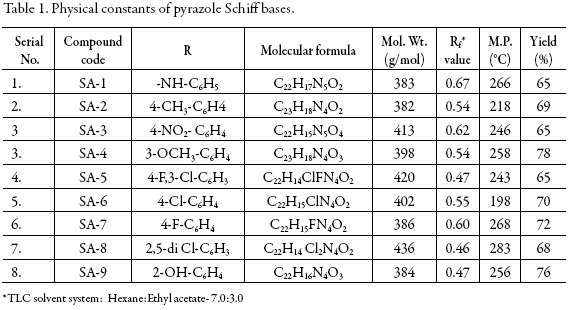
1. SA-1: (E)-3-(4-nitrophenyl)-1-phenyl-4-((2-phenylhydrazono)methyl)-1Hpyrazole
2. SA-2: (E)-4-methyl-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)aniline
3. SA-3: (E)-4-nitro-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)aniline
4. SA-4: (E)-4-methoxy-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)aniline
5. SA-5: (E)-3-chloro-4-fluoro-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)aniline
6. SA-6: (E)-4-chloro-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)aniline
7. SA-7: (E)-4-fluoro-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)aniline
8. SA-8: (E)-2,5-dichloro-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)aniline
9. SA-9: (E)-2-(((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)amino) phenol.
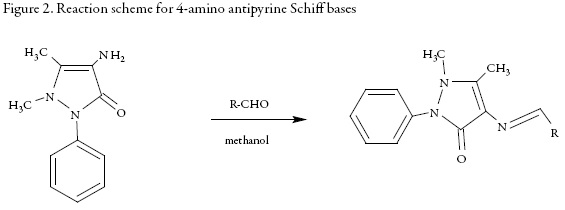
Synthesis of Schiff base from 4-amino antipyrine
Equimolar amount of different aldehydes and 4-amino anti pyridine was dissolved in 30 ml methanol. 0.1 mole of 4-amino antipyrine and few drops of glacial acidic acid were added in this solution and the mixture was refluxed for 12-14 hours at 80 °C-85 °C in water bath.
The resulting solution was cooled to room temperature and then poured in crushed ice with constant stirring. The product was filtered and washed with sodium bisulfate solution to remove the unreacted aldehyde. The crude product was crystallized from methanol and dried.
The following Schiff bases have been synthesized from 4-amino antipyrine:
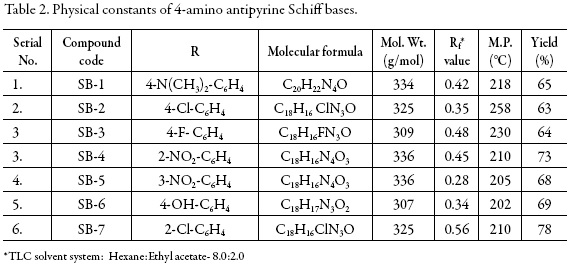
1. SB-1: (E)-4-((4-(dimethylamino)benzylidene)amino)1,5-dimethyl-2-phenyl1H-pyrazol-3(2H)-one
2. SB-2: (E)-4-((4-chlorobenzylidene)amino)1,5-dimethyl-2-phenyl-1H-pyrazol3(2H)-one
3. SB-3: (E)-4-((4-fluorobenzylidene)amino)1,5-dimethyl-2-phenyl-1H-pyrazol3(2H)-one
4. SB-4: (E)-1,5-dimethyl-4-((2-nitrobenzylidene)amino)-2-phenyl-1H-pyrazol3(2H)-one
5. SB-5: (E)-1,5-dimethyl-4-((3-nitrobenzylidene)amino)-2-phenyl-1H-pyrazol3(2H)-one
6. SB-6: (E)-4-((4-hydroxybenzylidene)amino)1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one
7. SB-7: (E)-4-((2-chlorobenzylidene)amino)1,5-dimethyl-2-phenyl-1H-pyrazol3(2H)-one.
All these compounds were synthesized according to the reaction schemes given in figures 1 and 2.
The physical parameters such as molecular formula, molecular weight, melting point, percentage yields, and Rf values along with the solvent system of all these synthesized compounds are given in tables 1 and 2 respectively.
The IR spectra (KBr pellets) were scanned on IR (SHIMADZU-FTIR-8400) over the frequency range from 4000-400 cm-1. 1H NMR spectra were scanned on Bruker Spectrometer (400 MHz) by using deuterated DMSO as a solvent. The Mass spectra were scanned on GCMS-SHIMADZU-QP2010.
Antibacterial activity
Test microorganisms
The synthesized compounds were tested for its antibacterial activity against four Gram positive bacteria viz. Bacillus cereus ATCC11778, Staphylococcus aureus ATCC 29737, Staphylococcus epidermidids ATCC 12228 and Micrococcus luteus ATCC10240, and three Gram negative bacteria viz. Proteus mirabilis NCIM2241, Escherichia coli ATCC25922 and Klebsiella aerogenes NCTC418. The microorganisms were obtained from National Chemical Laboratory (NCL), Pune, India. Microorganisms were maintained at 4 °C on nutrient agar slants.
Preparation of the test compound
For all the compounds, solutions were prepared at a concentration of 0.2 mg/ml in DMF.
Preparation of the plates and microbiological assay
The antibacterial evaluation was done by agar well diffusion method [29] using Mueller Hinton agar No. 2 as the nutrient medium. The agar well diffusion method was preferred to be used in this study since it was found to be better than the disc diffusion method as suggested by Essawi et al. [30]. The bacterial strains were activated by inoculating a loop full of test strain in 25 ml of N-broth and the same was incubated for 24 hours in an incubator at 37 oC. 0.2 ml of the activated strain was inoculated in Mueller Hinton agar.
Mueller Hinton agar kept at 45 oC was then poured in the Petri dishes and allowed to solidify. After solidification of the media, 0.85 cm ditch was made in the plates using a sterile cork borer and these were completely filled with the test solution. The plates were incubated for 24 h at 37 oC. The mean value obtained for the three wells was used to calculate the zone of growth inhibition of each sample. The controls were maintained for each bacterial strain. The inhibition zone formed by these compounds against the particular test bacterial strain determined the antibacterial activities of the synthetic compounds. The observed activities are also compared with well known antibiotics.
Results and discussion
The physical parameters all the synthesized compounds are given in tables 1 and 2.
Spectral data
SA-1: (E)-3-(4-nitrophenyl)-1-phenyl-4-((2-phenylhydrazono)methyl)-1H-pyrazole
IR (cm-1, KBr): 1543 (C=C str.), 1598 (C=N str. (Schiff base), 1543 (C=N str., (pyrazole moiety), 1256 (C-N str.), 961 (N-N str.), 1256 (N-O str.).
1H NMR (DMSO-d6) δ(ppm): 6.91- 7.02 (2H, doublet, Ar-H), 7.29- 7.34 (2H, doublet, Ar-H), 7.61-7.62 (2H, multiplet, Ar-H), 7.42-7.59 (9 H, multiplet, Ar-H), 7.63 (1 H, singlet, Ar-H), 8.85 (1H, singlet, -N=CH).
MS: (m/z) = 383
SA-2: (E)-4-methyl-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) aniline
IR (cm-1, KBr): 1537 (C=C str.), 1620 (C=N str. (Schiff base), 1598 (C=N str., (pyrazole moiety), 1256 (C-N str.), 961 (N-N str.), 1288 (N-O str.), 2922 (C-H str. (alkane).
1H NMR (DMSO-d6) δ(ppm): 3.75 (3H, singlet, C-CH3), 6.86-6.92 (2H, doublet, Ar-H), 7.32- 7.47 (2H, doublet, Ar-H), 7.51-7.63 (9 H, multiplet, Ar-H), 7.74 (1 H, singlet, Ar-H), 8.84 (1H, singlet, -N=CH).
MS: (m/z) = 382
SA-3: (E)-4-nitro-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) aniline
IR (cm-1, KBr): 1508 (C=C str.), 1635 (C=N str. (Schiff base), 1635 (C=N str., (pyrazole moiety), 1242 (C-N str.), 965 (N-N str.), 1339 (N-O str.).
1H NMR (DMSO-d6) δ(ppm): 6.64-6.71 (2H, doublet, Ar-H), 7.26- 7.32 (2H, doublet, Ar-H), 7.63-7.78 (9 H, multiplet, Ar-H), 7.95 (1 H, singlet, Ar-H), 8.67 (1H, singlet, -N=CH).
MS: (m/z) = 413
SA-4: (E)-4-methoxy-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) aniline
IR (cm-1, KBr): 1504 (C=C str.), 1627 (C=N str. (Schiff base), 1596 (C=N str., (pyrazole moiety), 1242 (C-N str.), 961 (N-N str.), 1338 (N-O str.), 1029 (C-O-C str. Ether).
1H NMR (DMSO-d6) δ(ppm): 3.85 (3H, singlet, C-CH3), 6.94-6.97 (2H, doublet, Ar-H), 7.23- 7.28 (2H, doublet, Ar-H), 7.39-7.55 (9 H, multiplet, Ar-H), 7.56 (1 H, singlet, Ar-H), 8.74 (1H, singlet, -N=CH).
MS: (m/z) = 398
SA-5: (E)-3-chloro-4-fluoro-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl) methylene)aniline
IR (cm-1, KBr): 1502 (C=C str.), 1598 (C=N str. (Schiff base), 1597 (C=N str., (pyrazole moiety), 1226 (C-N str.), 961 (N-N str.), 1340 (N-O str.), 1049 (C-F str.), 754 (C-Cl str.).
1H NMR (DMSO-d6) δ(ppm): 6.62-6.66 (1H, doublet, Ar-H), 7.07- 7.12 (1 H, triplet, Ar-H), 7.10-8.02 (10 H, multiplet, Ar-H), 8.37 (1 H, singlet, Ar-H), 8.70 (1H, singlet, -N=CH).
MS: (m/z) = 420
SA-6: (E)-4-chloro-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) aniline
IR (cm-1, KBr): 1540 (C=C str.), 1598 (C=N str. (Schiff base), 1635 (C=N str., (pyrazole moiety), 1336 (C-N str.), 960 (N-N str.), 1387 (N-O str.), 754 (C-Cl str.).
1H NMR (DMSO-d6) δ(ppm): 6.52-6.62 (2H, doublet, Ar-H), 7.01- 7.12 (2H, doublet, Ar-H), 7.33-7.52 (9 H, multiplet, Ar-H), 7.82 (1 H, singlet, Ar-H), 8.84 (1H, singlet, -N=CH).
MS: (m/z) = 402
SA-7: (E)-4-fluoro-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) aniline
IR (cm-1, KBr): 1500 (C=C str.), 1599 (C=N str. (Schiff base), 1637 (C=N str., (pyrazole moiety), 1333 (C-N str.), 961 (N-N str.), 1230 (N-O str.), 1079 (C-F str.).
1H NMR (DMSO-d6) δ(ppm): 6.45-6.53 (2H, doublet, Ar-H), 7.14- 7.26 (2H, doublet, Ar-H), 7.36-7.62 (9 H, multiplet, Ar-H), 7.96 (1 H, singlet, Ar-H), 8.85 (1H, singlet, -N=CH).
MS: (m/z) = 386
SA-8: (E)-2,5-dichloro-N-((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)aniline
IR (cm-1, KBr): 1521 (C=C str.), 1590 (C=N str. (Schiff base), 1628 (C=N str., (pyrazole moiety), 1328 (C-N str.), 962 (N-N str.), 1248 (N-O str.), 751 (C-Cl str.).
1H NMR (DMSO-d6) δ(ppm): 6.43-6.51 (1H, doublet, Ar-H), 7.14- 7.20 (1 H, triplet, Ar-H), 7.35-8.24 (10 H, multiplet, Ar-H), 8.28 (1 H, singlet, Ar-H), 8.96 (1H, singlet, -N=CH).
MS: (m/z) = 436
SA-9: (E)-2-(((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)amino) phenol
IR (cm-1, KBr): 1515 (C=C str.), 1585 (C=N str. (Schiff base), 1634 (C=N str., (pyrazole moiety), 1332 (C-N str.), 960 (N-N str.), 1241 (N-O str.).
1H NMR (DMSO-d6) δ(ppm): 6.42-6.56 (2H, doublet, Ar-H), 7.08- 7.14 (2H, doublet, Ar-H), 7.25-7.63 (9 H, multiplet, Ar-H), 7.68 (1 H, singlet, Ar-H), 7.92 (1H, singlet, -OH), 8.89 (1H, singlet, -N=CH).
MS: (m/z) = 384
SB-1: (E)-4-((4-(dimethylamino)benzylidene)amino)1,5-dimethyl-2-phenyl-1Hpyrazol-3(2H)-one
IR (cm-1, KBr): 2930 (-C-H str. (asym.)), 2804 (-C-H str. (sym.)), 1582 (C=C str.) 1648 (C=N str.), 1648 (C=O str.), 1582 (C=N str.), 1290 (C-N str.), 973 (N-N str.).
1H NMR (DMSO-d6) δ(ppm): 2.11-2.35 (6H, singlet, C-CH3), 2.56 (3H, singlet, C-CH3), 3.25 (3H, singlet, N-CH3), 7.05- 7.10 (2H, triplet, Ar-H), 7.35-7.39 (1H, triplet, Ar-H),7.47-7.94 (6H, multiplet, Ar-H), 9.85 (1H, singlet, N=CH-).
MS: (m/z) = 334
SB-2: (E)-4-((4-chlorobenzylidene)amino)1,5-dimethyl-2-phenyl-1H-pyrazol3(2H)-one
IR (cm-1, KBr): 2939 (-C-H str. (asym.)), 2850 (-C-H str. (sym.)), 1571 (C=C str.) 1571 (C=N str.), 1650 (C=O str.), 1593 (C=N str.), 1290 (C-N str.), 739 (C-Cl str.).
1H NMR (DMSO-d6) δ(ppm): 1H NMR (DMSO-d6) δ(ppm) : 2.32 (3H, singlet, C-CH3), 3.22 (3H, singlet, N-CH3), 6.91- 6.96 (2H, triplet, Ar-H), 7.20-7.29 (1H, triplet, Ar-H),7.35-7.55 (6H, multiplet, Ar-H), 9.74 (1H, singlet, N=CH-).
MS: (m/z) = 325
SB-3: (E)-4-((4-fluorobenzylidene)amino)1,5-dimethyl-2-phenyl-1H-pyrazol3(2H)-one
IR (cm-1, KBr): 2950 (-C-H str. (asym.)), 2830 (-C-H str. (sym.)), 1568 (C=C str.) 1597 (C=N str.), 1651 (C=O str.), 1597 (C=N str.), 1454 (C-H str.), 1221 (C-F str.).
1H NMR (DMSO-d6) δ(ppm): 2.47 (3H, singlet, C-CH3), 3.15 (3H, singlet, N-CH3), 7.07- 7.11 (2H, triplet, Ar-H), 7.30-7.34 (1H, triplet, Ar-H),7.38-7.85 (6H, multiplet, Ar-H), 9.70 (1H, singlet, N=CH-).
MS: (m/z) = 309
SB-4: (E)-1,5-dimethyl-4-((2-nitrobenzylidene)amino)-2-phenyl-1H-pyrazol3(2H)-one
IR (cm-1, KBr): 2945 (-C-H str. (asym.)), 2835 (-C-H str. (sym.)), 1568 (C=C str.) 1591 (C=N str.), 1647 (C=O str.), 1591 (C=N str.), 1381 (N-O str.).
1H NMR (DMSO-d6) δ(ppm): 2.48 (3H, singlet, C-CH3), 3.21 (3H, singlet, N-CH3), 7.28- 7.32 (2H, triplet, Ar-H), 7.38-7.40 (1H, triplet, Ar-H),7.17-7.46 (6H, multiplet, Ar-H), 9.90 (1H, singlet, N=CH-).
MS: (m/z) = 336
SB-5: (E)-1,5-dimethyl-4-((3-nitrobenzylidene)amino)-2-phenyl-1H-pyrazol3(2H)-one
IR (cm-1, KBr): 2942 (-C-H str. (asym.)), 2815 (-C-H str. (sym.)), 1584 (C=C str.) 1599 (C=N str.), 1662 (C=O str.), 1590 (C=N str.), 1372 (N-O str.).
1H NMR (DMSO-d6) δ(ppm): 1H NMR (DMSO-d6) δ(ppm) : 2.41 (3H, singlet, C-CH3), 3.07 (3H, singlet, N-CH3), 6.71- 6.80 (2H, triplet, Ar-H), 7.21-7.39 (1H, triplet, Ar-H),7.52-7.80 (6H, multiplet, Ar-H), 9.99 (1H, singlet, N=CH-).
MS: (m/z) = 336
SB-6: (E)-4-((4-hydroxybenzylidene)amino)1,5-dimethyl-2-phenyl-1H-pyrazol3(2H)-one
IR (cm-1, KBr): 2945 (-C-H str. (asym.)), 2835 (-C-H str. (sym.)), 1584 (C=C str.) 1585 (C=N str.), 1646 (C=O str.), 3350 (O-H str.).
1H NMR (DMSO-d6) δ(ppm): 1H NMR (DMSO-d6) δ(ppm) : 2.46 (3H, singlet, C-CH3), 3.11 (3H, singlet, N-CH3), 6.85- 6.87 (2H, triplet, Ar-H), 7.30-7.32 (1H, triplet, Ar-H),7.38-7.70 (6H, multiplet, Ar-H), 9.21 (1H, singlet, -OH), 9.85 (1H, singlet, N=CH-).
MS: (m/z) = 307
SB-7: (E)-4-((2-chlorobenzylidene)amino)1,5-dimethyl-2-phenyl-1H-pyrazol3(2H)-one
IR (cm-1, KBr): 2940 (-C-H str. (asym.)), 2835 (-C-H str. (sym.)), 1555 (C=C str.) 1588 (C=N str.), 1650 (C=O str.), 1588 (C=N str.), 750 (C-Cl str.).
1H NMR (DMSO-d6) δ(ppm): 2.389 (3H, singlet, -CH3), 1.713-2.796 (4H, multiplet, C-H), 6.170 (1H, singlet, -CH), 7.196-7.936 (8H, multiplet -CH), 10.201 (1H, singlet, -NH).
MS: (m/z) = 307
Antibacterial activity
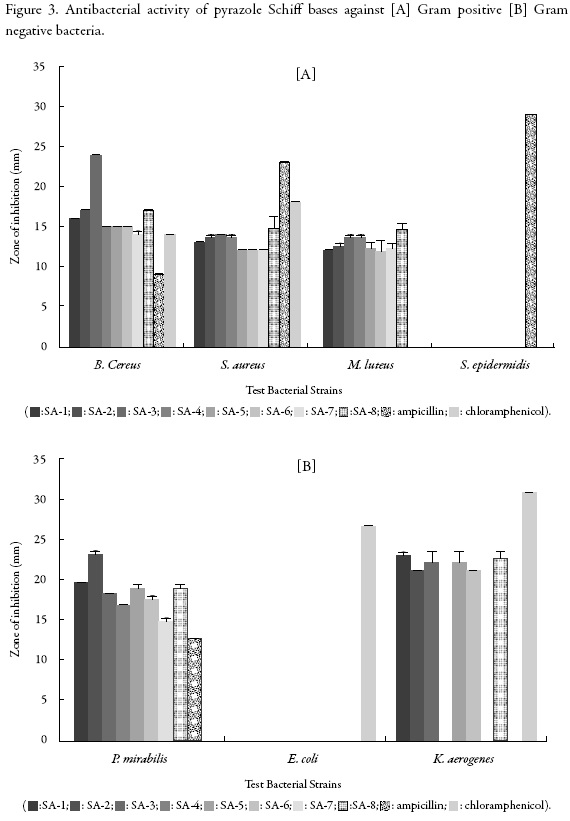
The inhibition zone of pyrazole Schiff bases against different Gram positive and Gram negative bacteria are given in Figure 3.
It is evident from figure 3 [A] that SA-3 and SA-8 exhibited maximum inhibition against all the selected Gram positive bacteria except S. epidermidis. Against B. cereus, minimum inhibition is exhibited by SA-7 while other compounds show almost equal inhibition. Against S. aureus, SA-3 and SA-8 shows maximum inhibition followed by SA-2 and SA-4. Minimum inhibition is exhibited by SA-1, SA-5, SA-6 and SA-7. Against M. luteus also, SA-8 showed maximum inhibition followed by SA-3 and SA-4. SA-6 exhibited minimum inhibition. For S. epidermidis, not a single compound showed inhibition. Thus, S. epidermidis is the most resistant strain.
Thus, for the studied Gram positive bacteria, different compounds behave differently. All the compounds have the same common moiety but different substituents as side chain as shown in table 1. SA-3 and SA-8, which exhibited maximum inhibition, contain 4-nitro and 2, 5-dichloro aniline respectively as side chain. Thus, these two groups are the most effective substituent groups for the selected Gram positive bacteria.
Figure 3 [B] shows that against P. mirabilis, SA-2 shows maximum inhibition followed by SA-1, SA-5 and SA-8 and SA-7 shows minimum activity. SA-2 contains 4-methyl aniline side chain, which is found to enhance the inhibition whereas 4-fluoro aniline (as in SA-7) is least effective. Against E. coli, none of the compound exhibited activity indicating thereby that it is the most resistant bacteria strain.
Against K. aerogenes, SA-1 showed maximum inhibition which contains phenyl hydrazine. This is followed by SA-8 containing 2, 5-dichloro aniline while SA-4 and SA-7 showed no inhibition at all. Thus, the presence of 4-methoxy (as in SA-4) and 4-fluoro (as in SA-7) had no effect on these selected bacterial strains.
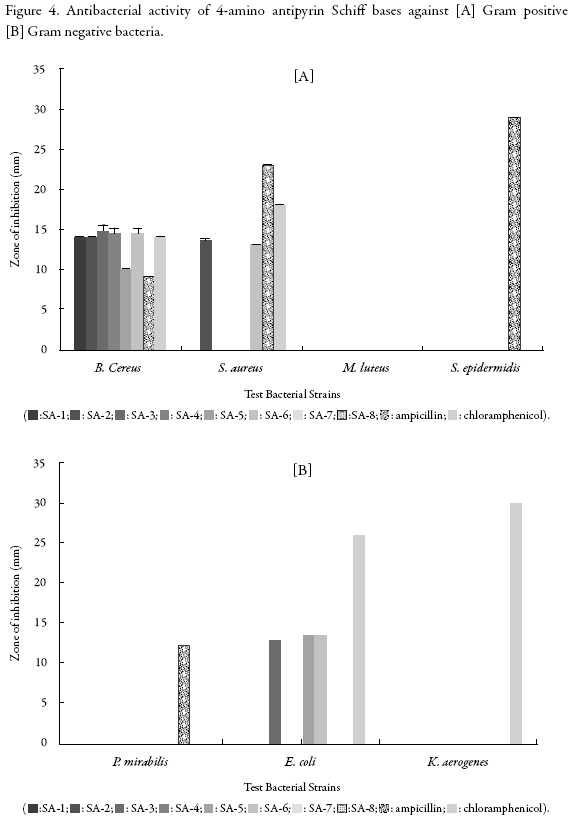
The inhibition zone of 4-amino antipyrine Schiff bases against different Gram positive and Gram negative bacteria are given in Figure 4.
It is evident from Figure 4 [A] that against B. cereus, all the compounds except SB-7 exhibited inhibition and maximum inhibition is for SB-3 which is followed by SB-6. SB-5 showed minimum inhibition. Against S. aureus, only SB-2 and SB-6 exhibited inhibition. Other compounds had no effect on this strain. For M. luteus and S. epidermidis, none of the compounds showed inhibition. Thus, these two strains are most resistant for these compounds.
Accordingly, again different compounds behave differently for different bacteria. The compounds have the same common moiety but different substituents as side chain which is given in table 2. SB-3 contains 4-fluoro phenyl whereas SB-6 is having 4-hydroxy phenyl. Thus, these two groups are more effective against B. cereus than other substitution groups. 2-chloro phenyl present in SB-7 is not effective at all against this bacterial strain. Against S. aureus, only 4-chloro phenyl (as in SB-2) and 4-hydroxy phenyl (as in SB-6) are effective. However, when chloro group is at 2nd position (as in SB-7), there is no inhibition. This suggests that position plays an important role in inhibition. Thus, out of four selected Gram positive bacterial strains, M. luteus and S. epidermidis are the most resistant Gram positive bacterial strain.
Figure 4 [B] shows that only compounds, SB-3, SB-5 and SB-6 could inhibit E. coli. Other compounds had no effect against this bacterial strain. This suggests that 4-fluoro phenyl, 3-nitro phenyl and 4-hydroxy phenyl are effective against this strain. SB-4 also contains nitro group but at 2nd position but there is no effect of this compound on E. coli. Thus, the presence of group at this position is not effective. Not a single compound could inhibit P. mirabilis and K. aerogenes. Thus, P. mirabilis and K. aerogenes are the most resistant Gram positive bacterial strain.
Comparison of inhibition exhibited by Schiff bases of these two different moieties shows that most of the pyrazole Schiff bases showed inhibition. Schiff bases are not effective against S. epidermidis. Further, the extent of inhibition is higher for pyrazole Schiff bases. Thus, pyrazole Schiff bases are better for inhibition for these selected Gram positive and Gram negative bacterial strains.
Conclusion
It is concluded that the inhibition depends on the type and position of substitutions of the compounds. The effect of different substitution group is different when attached to different moiety. The pyrazole Schiff bases are better for inhibition for these selected Gram positive and Gram negative bacterial strains in comparison to 4-amino antipyrine. Overall, B. cereus is the most susceptible bacteria.
Acknowledgements
The authors are very much thankful to the Head of Chemistry Department of Saurashtra University for providing necessary facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
1. N.R. Bader, Applications of Schiff 's bases chelates in quantitative analysis: a review, Rasayan J. Chem., 3(4), 660-670 (2010). [ Links ]
2. S. Çakır, E. Biçer, Synthesis, spectroscopic and electrochemical characteristics of a novel Schiff-base from saccharin and tryptophan, J. Iran. Chem. Soc., 7(2), 394404 (2010). [ Links ]
3. S. Kumar, D.N. Dahr, P.N. Saxena, Applications of metal complexes of Schiff bases. A review, J. Sci. Ind. Res., 68, 181-187 (2009). [ Links ]
4. P. Przybylski, A. Huczyński, K. Pyta, B. Brzezinski, F. Bartl, Biological properties of Schiff bases and azo derivatives of phenol, Curr. Org. Chem., 13, 124-148 (2009). [ Links ]
5. C. Chandramouli, M.R. Shivanand, T.B. Nayanbhai, B. Bheemachari, R.H. Udupi, Synthesis and biological screening of certain new triazole Schiff bases and their derivatives bearing substituted benzothiazole moiety, J. Chem. Pharm. Res., 4(2), 1151-1159 (2012). [ Links ]
6. S.S. Abd Rehim, M.A.M. Ibrahim, K.F. Khalid, The inhibition of 4-(2- amino-5methyl phenylazo)antipyrine on corrosion of mild steel in HCl solution, Mater. Chem. Phys., 70, 268-273 (2001). [ Links ]
7. E. Yousif, N. Salih, J. Salimon, Improvement of photo stabilisation of PVC in the presence of 2N-salisylidine-5-(substituted)-1,3,4-triadiazole, J. Appl. Polym. Sci., 120, 2207-2214 (2011). [ Links ]
8. E. Yousif, J. Salimon, N. Salih, New stabilizer for polystyrene based on 2N-salisylidine-5-(substituted)-1,3,4-triadiazole compounds, J. Saudi Chem. Soc., 16, 299-306 (2012). [ Links ]
9. K.M. Abuamer, A.A. Maihub, M.M. El-Ajaily, A.M. Etorki, M.M. Abou-Krisha, A. Almagani, The role of aromatic Schiff bases in the dyes techniques, Int. J. Org. Chem., 4, 7-15 (2014). [ Links ]
10. S. Mallad, A.M. Isloor, S. Isloor, D.S. Akhila, H.K. Fun, Synthesis, characterization and antibacterial activity of some new pyrazole based Schiff basas, Arab. J. Chem., 6(3), 335-340 (2013). [ Links ]
11. S.A. Patil, C .T. Prabhakar, B.M. Halasangi, S.S. Toragalmathand, P.S. Badami, DNA cleavage, antibacterial, antifungal anthelmintic studies of Co (II), Ni (II) and Cu (II) complexes of coumarin Schiff bases: synthesis and spectral approach, Spectrochim. Acta A Mol. Biomol. Spectrosc., 137, 641-651 (2014). [ Links ]
12. Z. Guo, R. Xing, S. Liu, Z. Zhong, X. Ji, L. Wang, Antifungal properties of Schiff bases of chitosan N-substituted chitosan and quaternized chitosan, Carbohydr. Res., 42(10), 1329-1332 (2007). [ Links ]
13. N. Raman, S. Thalamuthu, J. Dhaveethuraja, M.A. Neelakandan, S. Banerjee, DNA cleavage and antimicrobial activity studies on transition metal (ii) complexes of 4-amino antipyrine derivative, J. Chil. Chem. Soc., 53(1), 1450-1454 (2008). [ Links ]
14. K.S. Kumar, S. Ganguly, R. Veerasamy, E. de Clercq, Synthesis, antiviral activity and cytotoxic evolution, Eur. J. Med. Chem., 45(11), 5474-5479 (2010). [ Links ]
15. M.S. Alam, J.H. Choi, D.U. Lee, Synthesis of novel Schiff base analogues of 4-amino-1,5-dimethyl-2-phenylpyrazol-3-one and their evaluation for antioxidant and anti-inflammatory activity, Bioorg. Med. Chem., 20, 4103-4108 (2012). [ Links ]
16. B.S. Sathe, E. Jaychandran, V.A. Jagtap, G.M. Sreenivasa, Synthesis characterization and anti-inflamatory evolution of new benzofluorothiazole Schiff 's bases, Int. J. Pharm. Res. Dev., 3(3), 164-169 (2011). [ Links ]
17. A. Pandey, D. Dewangan, S. Verma, A. Mishra, R.D. Dubey, Synthesis of Schiff bases of 2-amino-5-aryl-1,3,4-thiadiazole and its analgesic, antiinflammatory, antibacterial and anti-tubercular activity, Int. J. ChemTech Res., 3(1), 178-184 (2011). [ Links ]
18. R.P. Chinnasamy, R. Sundararajan, S. Govindaraj, Synthesis, characterization, and analgesic activity novel schiff base of isatin derivatives, J. Adv. Pharm. Tech. Res., 1(3), 342-347 (2010). [ Links ]
19. G.S. Kurdekar, M.P. Sathishma, S. Budagumpi, 4-Amino antipyrine-based Schiff-base transition metal complexes as potent anticonvulsant agents, Med. Chem. Res., 21, 2273-2279 (2012). [ Links ]
20. T. Aboul-Fadl, F.A. Mohammed, E.A. Hassan, Synthesis, antitubercular activity and pharmacokinetic studies of some schiff bases derived from 1- alkylisatin and isonicotinic acid hydrazide, Arch. Pharm. Res., 26(10), 778-784 (2003). [ Links ]
21. S.M.M. Ali, M.A. Azad, M. Jesmin, In vivo anticancer activity of vanillin semicarbazone, Asian Pacific J. Tropical Biomed., 2(6), 438-442 (2012). [ Links ]
22. N.M. Aburas, N.R. Stevanović, M. Milčić, A.Đ. Lolić, M.M. Natić, Ž.L. Tešić, R.M. Baošić, Influence of the structure on the antioxidant activity of tetradentate Schiff bases and their copper(II) complexes: Possible mechanisms, J. Braz. Chem. Soc., 24(8), 1322-1328 (2013). [ Links ]
23. Z. Guo, R. Xing, S. Liu, H. Yu, P. Wang, C. Li, P. Li, The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan, Bioorg. Med. Chem. Lett., 15(20), 4600-4603 (2005). [ Links ]
24. T. Singh, D. Khobragade, Synthesis of thiazolidine-4-one for their anthelmintic activity, Unique J. Pharma. Biol. Sci., 2(1), 13-15 (2014). [ Links ]
25. Y. Li, Z. S. Yang, H. Zhang, B.J. Cao, F.D. Wang, Artemisinin derivatives bearing Mannich base group: synthesis and antimalarial activity, Bioorg. Med. Chem., 11, 4363-4368 (2003). [ Links ]
26. M.S. Alam, D.U. Lee, M.L. Bari, Antibacterial and cytotoxic activities of Schiff base analogues of 4-aminoantipyrine, J. Korean Soc. Appl. Biol. Chem., 57(5), 613-619 (2014). [ Links ]
27. S. Baluja, S. Chanda, N. Godvani, Synthesis and antibacterial studies of some metal chelates of 1,2,4-triazole Schiff bases, Pharm. Chem. J., 48(12), 795-799 (2015). [ Links ]
28. D. Menpara, K. Nandha, S. Baluja, S. Chanda, Antimicrobial activity of some quinazoline heterocycles, World J. Pharm. Sci., 2(8), 860-865 (2014). [ Links ]
29. J. Parekh, P. Inamdar, R. Nair, S. Baluja, S. Chanda, Synthesis and antibacterial activity of some Schiff bases derived from 4-amino benzoic acid, J. Serb. Chem. Soc., 70, 1155-1162 (2005). [ Links ]
30. T. Essawi, M. Srour, Screening of some Palestinian medicinal plants for antibacterial activity, J. Ethnopharmacol., 70, 343-349 (2000). [ Links ]
How to cite this article
S. Baluja, S. Chanda, Synthesis, characterization and antibacterial screening of some Schiff bases derived from pyrazole and 4-amino antipyrine, Rev. Colomb. Cienc. Quím. Farm., 45(2), 201-218 (2016).