Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418
Rev. colomb. cienc. quim. farm. vol.45 no.3 Bogotá Sep./Dec. 2016
https://doi.org/10.15446/rcciquifa.v45n3.62014
http://dx.doi.org/10.15446/rcciquifa.v45n3.62014
Ultrasonic behavior of various chalcones in some solvents at different temperatures
Comportamiento ultrasónico de varias chalconas en algunos disolventes a diferentes temperaturas
Shipra Baluja*, Divyata Lava
Physical Chemistry Laboratory, Department of Chemistry, Saurashtra University, Rajkot-360005, India
* E-mail: shipra_baluja@rediffmail.com
Received: April 13, 2016 Accepted: August 29, 2016
Summary
Ultrasonic velocity, density and viscosity of some synthesized chalcones were measured in N,N-dimethyl formamide and chloroform at different temperatures (298.15 to 318.15 K). From these experimental data, various acoustical parameters such as specific impedance (Z), adiabatic compressibility (ks), Rao's molar sound function (Rm), intermolecular free path length (Lf), solvation number (Sn), internal pressure (Π) have been calculated in order to understand the molecular interactions in the studied solutions. The results are interpreted in terms of molecular interactions occurring in the solutions.
Keywords: Ultrasonic velocity, chalcones, acoustical parameters, DMF, chloroform.
Resumen
La velocidad ultrasónica, la densidad y la viscosidad de soluciones de algunas chalconas sintéticas se midieron en N, N-dimetilformamida y cloroformo a diferentes temperaturas (desde 298,15 hasta 318,15 K). A partir de estos datos experimentales, se calcularon diversos parámetros acústicos tales como la impedancia específica (Z), la compresibilidad adiabática (ks), la función de sonido molar de Rao (Rm), la longitud de trayecto libre intermolecular (Lf), el número de solvatación (Sn) y la presión interna (Π), para comprender las interacciones moleculares en las soluciones estudiadas. Los resultados se interpretan en términos de las posibles interacciones moleculares que ocurren en las soluciones.
Palabras clave: Velocidad ultrasónica, chalconas, parámetros acústicos, DMF, cloroformo.
Introduction
Ultrasonic, a versatile non-destructive technique is a subject of extensive research because of its applications in various fields such as biology, medicine, in chemical industries, consumer industries, medical field, physics, etc. [1-9]. Further, now a days, ultrasonic waves are used in chemical and food processing industries [10-13].
The study of ultrasonic velocity along with density and viscosity at different temperatures and concentrations has been used to draw conclusion about molecular interactions, complex formation, etc. [14-16]. The ultrasonic technique is highly sensitive to molecular interactions and it gives valuable information about nature and strength of molecular interactions in solutions.
Chalcones are an important class of biologically active compounds which are known to exhibit a wide spectrum of biological and pharmacological activities like antispasmodic, anti-helmintics, anti-inflammatory, antiviral, ant allergic, antifungal, antibacterial, anticancer, anti-tubercular, anti-HIV, antioxidant, etc. [17-27].
Owing to vast biological and pharmacological applications of chalcones, it would be interesting to study acoustical properties of such compounds. The obtained data may be useful to scientists for Quantitative Structure Activity Relationship (QSAR) study of these compounds.
Thus, in the present work, some new chalcones are synthesized and their structure characterization was done by IR, 1H NMR and mass spectral data. The density, ultrasonic velocity and viscosity of these synthesized compounds were measured in N,N-dimethyl formamide and chloroform over a wide range of concentration at different temperatures. From these experimental data, various acoustical parameters such as specific impedance (Z), adiabatic compressibility (xs), Rao's molar sound function (Rm), inter molecular free path length (Lf), solvation number (Sn), internal pressure (Π) have been calculated. The obtained results are discussed in term of solute-solute and solute-solvent interactions occurring in the studied solutions.
Experimental
Materials
α-tetralone, methanol, 4-fluoro benzaldehyde and 4-methyl benzaldehyde used in the synthesis were supplied from Spectrochem Pvt. Ltd. (Mumbai, India). The solvents, DMF and chloroform used in the present work were of AR grade supplied by LOBA Chemie Pvt. Ltd. (Mumbai, India) and were purified according to the standard reported method [28].The purified solvents were kept over molecular sieves. The final purities of the solvents were checked by GC-MS (SHIMADZU-Model No.-QP-2010).
Synthesis
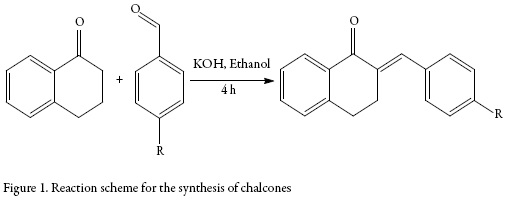
Equimolar mixture of α-tetralone and substituted benzaldehydes in ethanol was refluxed for 4 h in presence of catalytic amount of potassium hydroxide. The reaction progress was checked by analytical thin layer chromatography (TLC) using (4:1-Hexane: Ethyl acetate) as mobile phase. After completion of reaction, the temperature of reaction mass was allowed cool up to room temperature and the resulting solid was filtered, washed with water and was dried under vacuum to give crude product. The obtained crude product was purified by washing with diethyl ether.
The reaction scheme is given in figure 1.
Overall, two compounds were synthesized and the IUPAC names of these compounds are:
SB-1:2-(4-fluorobenzylidine)-3,4-dihydro napthalene-1,(2H)-one.
SB-2:2-(4-methylbenzylidine)-3,4-dihydro napthalene-1,(2H)-one.
Spectroscopy study
The structure of the synthesized compounds was confirmed by IR, 1H NMR and mass spectral analysis. The IR spectra of compounds were taken on FT-IR (SHIMADZU Model-IRaffinity-1S). 1H NMR spectra were taken on a Bruker AVANCE III (400 MHz). In all the cases, 1H NMR spectra were obtained in deuterated dimethyl sulf-oxide (DMSO-d6) using TMS as an internal standard. The NMR signals are reported in δ ppm. Mass spectra were determined using direct inlet probe on a GC-MS (SHI-MADZU Model-QP2010) mass spectrometer.
The melting points of compounds were measured by Different Scanning Calorimeter (SHIMADZU DSC-60) under nitrogen atmosphere.
Measurements of density, ultrasound velocity and viscosity
Measurements of density and ultrasound velocity
The solutions of chalcones were prepared in DMF and chloroform in the concentration range from 0.01 to 0.10 mol.L-1and were stored in bottles with PTFE septum until further use. The standard uncertainties of solutions are 0.0001 mol.L-1. An electronic balance (Mettler Toledo Model-AB204-S) with accuracy of ±0.0001 g was used for weighing.
The density and ultrasonic velocity of pure solvents and solutions were measured at different temperatures (298.15, 308.15 and 318.15 K) using Anton Paar Density and Sound velocity meter (DSA 5000M). The temperature was controlled up to ± 0.01 K by a built-in Peltier device. Before measurements, the instrument DSA 5000M was calibrated by ultra-pure water in the experimental temperature range. The standard uncertainty of the density and ultrasonic velocity was found to be 0.5 kg.m-3 and 0.5 m.s-1, respectively.
Measurement of viscosity
To determine the viscosity of pure solvents and solutions, Ubbelohde viscometer was used. The measured quantity of the distilled water / solvent / solution was placed in the viscometer, which was suspended in a constant temperature bath (NOVA Instruments Pvt. Ltd.-Ahmedabad) (NV-8550 E) at definite temperature. The accuracy of bath was ± 0.5° C. A digital stop watch (Hanhart-Germany) with accuracy of ± 0.01 second was used to determine flow time of solutions. Using the flow times (t) and known viscosity of standard water sample, the viscosity of solvent and solutions were determined according to equation:
where η1 and η)2 are the viscosities of water and solutions respectively; t1 and ρ1 are the flow of time and density of water whereas t2 and p2 are the flow of time and density of solutions respectively.
Results and Discussion
Table 1 shows the substitutions and other physical properties of synthesized compounds.
Spectral Data:
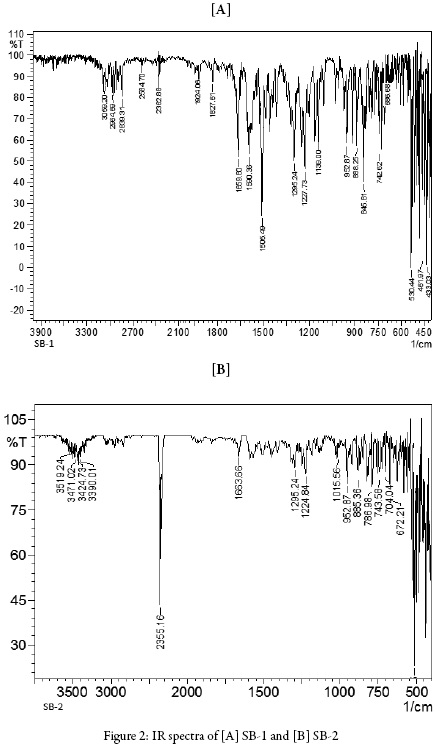
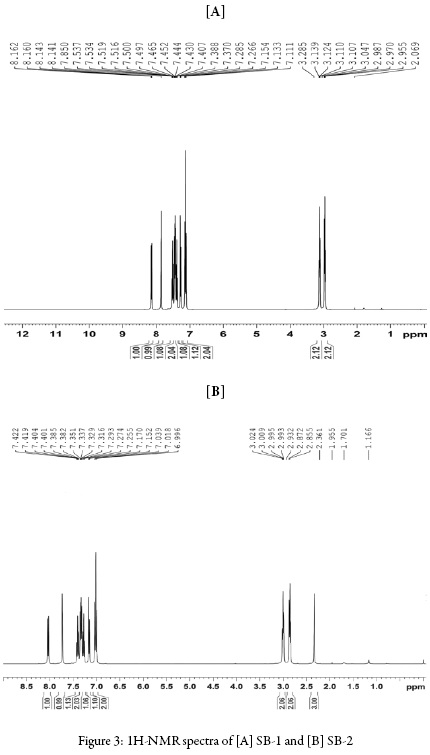
Figures 2 and 3 show IR and 1H NMR of synthesized compounds.
SB-1:
IR (cm-1, KBr): 3059.20 (Ar-H asym. stretching), 2964.69-2839.31 (CH2 stretching of cyclohexanone ring), 1659.80(C = O stretching), 1295.24-1227.73 (-CH bending), 952.87 (ring stretching in cyclohexanone), 845 (C-H out of plane bending), 1139 (C-F stretching).
1H NMR (CDCl3) δ(ppm): 2.955-2.987 (2H, triplet, J = 6.4), 3.107-3.139 (2H, triplet, J =6.4), 7.111-7.154 (2H, triplet, J = 8.6), 7.266-7.285 (1H, doublet, J = 7.6), 7.370-7.389 (1H,triplet, J = 3.8), 7.408-7.466, (2H, quartet), 7.497-7.537 (1H, triplet, J = 8.0), 7.851 (1H, Singlet), 8.141-8.162 (1H, doublet, J = 8.4).
MS: (m/z) = 252.12
SB-2:
IR (cm1, KBr): 3050.52 (Ar-H asym. str.), 2846.06 (CH stretching of cyclohexanone ring), 1663.80 (C = O stretching), 1295.24-1224.73 (-CH2 bending), 952.87 (ring stretching in cyclohexanone), 845 (C-H out of plane bending), 2951.19 (-CH3 stretching).
1HNMR (CDCl3) d(ppm): 2.361 (3H, singlet) 2.855-2.932 (2H, triplet, J = 6.4), 2.993-3.024 (2H, triplet, J = 6.4), 6.996-7.152 (2H, triplet, J = 8.6), 7.170-7.255 (1H, doublet, J = 7.6), 7.274-7.316 (1H, triplet, J = 3.8), 7.329-7.351 (2H, quartet), 7.3827.385 (1H, triplet, J = 8.0), 7.401-7.404 (1H, Singlet), 7.419-7.422 (1H, doublet, J = 8.4).
MS: (m/z) = 248.32
Table 2 shows experimental density, ultrasonic velocity and viscosities of pure solvents and solutions of both the compounds. From the experimental data of density, viscosity and ultrasonic velocity, various acoustical parameters such as specific acoustical impedance (Z), adiabatic compressibility (ks), intermolecular free path length (Lf), Rao's molar sound function (Rm), free volume (Vf), internal pressure (Π), solvation number (Sn) etc. are evaluated using following equations:
Specific acoustical impedance (Z):
Adiabatic compressibility (xs):
Intermolecular free path length (Lf )[29]:
where Kj is Jacobson constant (=2.0965 x 10-6)
Rao's molar sound function ( Rm ) [30]:
where M is the apparent molecular weight of solution and can be calculated according to the following equation:
where W1 and W2 are weight fractions of solvent and solute respectively. M1 and M2 are molecular weights of the solvent and solute respectively.
Free volume (Vf) [31]:
Internal pressure (Π) [32]:
Solvation number (Sn) [31]:
where X is the number of grams of solute in 100 g of the solution. M1 and M2 are the molecular weights and Xa1 and Xa are adiabatic compressibility of solvent and solute respectively.
Some of these evaluated parameters are reported in table 3.
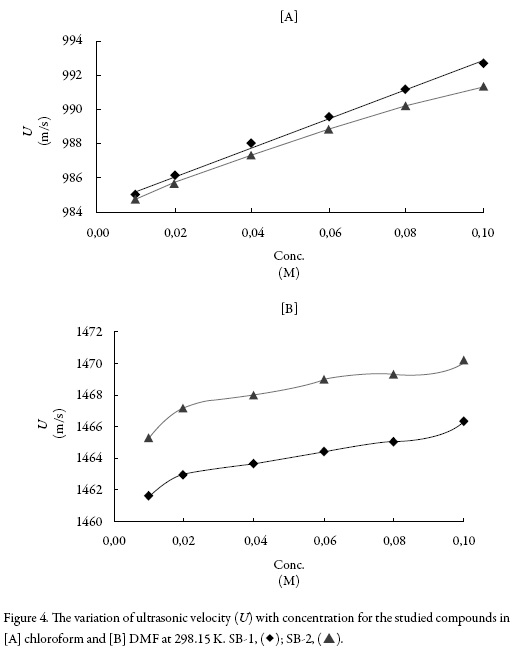
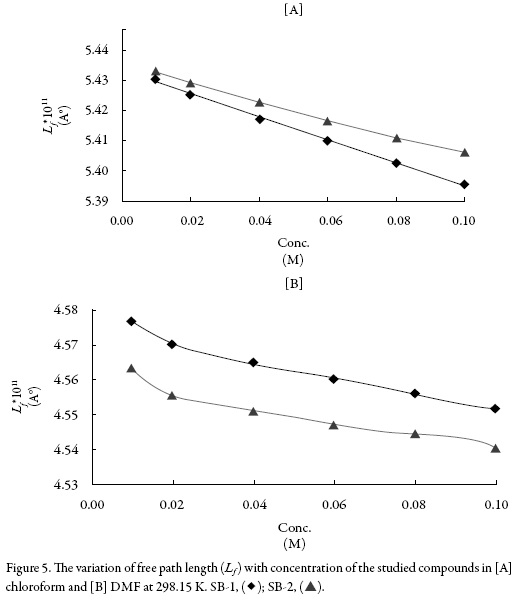
Figure 4 shows the variation of ultrasonic velocity with concentration for the studied compounds in both the solvents. It is observed from Figure 4 that the velocity increases almost linearly with concentration for both SB-1 and SB-2 in chloroform whereas in dimethyl formamide, the increase is non-linear. Further, the velocity is higher in DMF than that in chloroform. The increase of velocity is related to intermolecular free path length (Lf) which is found to decrease with concentration in both the solvents as shown in Figure 5. Again, the decrease is linear in chloroform and non-linear in dimethyl for-mamide for both the compounds. Thus, velocity is reciprocal of intermolecular free path length. The decrease of Lf suggests that compound and solvent molecules interact with each other i.e., compound-solvent interactions takes place in solution. This causes decrease in distance between compound and solvent molecules causing thereby an increase in velocity.
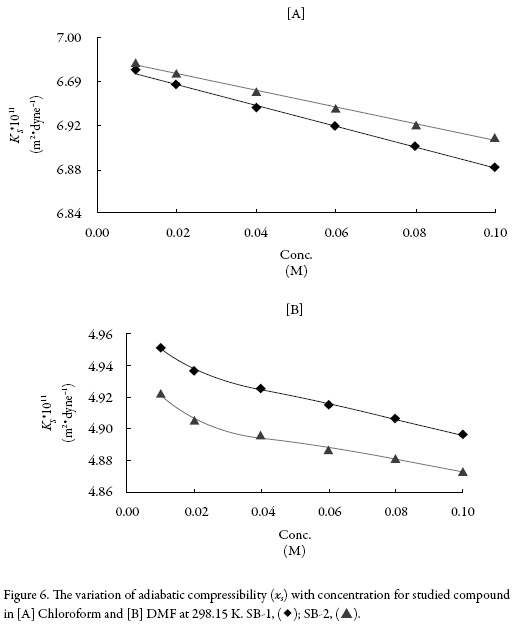
Figure 6 shows that the variation of adiabatic compressibility (ks) with concentration which is again found to decrease with concentration for both compounds in both the solvents. This further confirms the existence of compound-solvent interactions in studied systems. The predominance of solute-solvent interaction is further proved by increase of specific impedance (Z) (table 3).
Table 3 shows that Rao's molar function increases continuously for all the solutions for both the compounds indicating thereby that there is no complex formation in the studied solutions. The internal pressure (Π) is a measure of cohesive energy in the solution which is found to decrease. This suggests that in the studied systems, compound-compound interactions also exist. This is again suggested by increase in free volume which is found to increase. However, the decrease of internal pressure and increase in free volume is in very small magnitude. Thus, in the studied systems, both compound-solvent and compound-compound interactions are present but their magnitudes are much different.
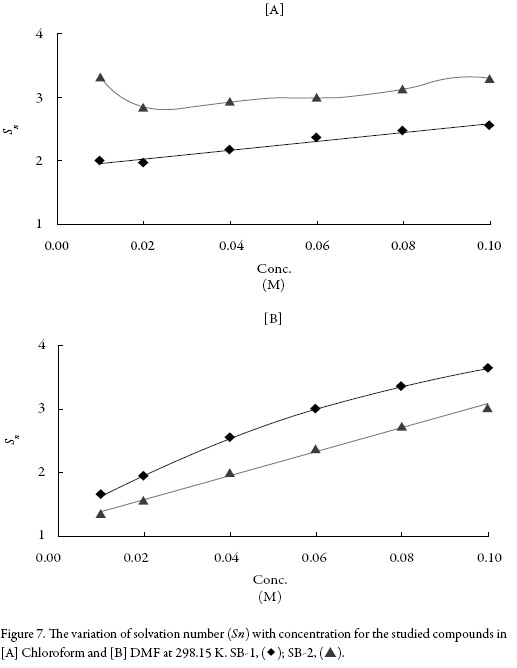
The magnitude and type of interactions are also predicted by a parameter Solvation number which is a measure of structure forming or structure breaking tendency of solute in a particular solvent. Figure 7 shows the variation of solvation number (Sn) with concentration. It is observed from figure 7 that solvation number increases with concentration for both the compounds and are found to be positive. The positive values of solvation number suggest structure forming tendency of the studied compounds in the studied solvents. This again proves that in studied solutions, compound-solvent interactions dominate.
Conclusion
On the bases of the evaluated data, it is concluded that in both chloroform and DMF, predominance of compound-solvent interactions exist for both the compounds. Further, interactions increases with increase in concentration but decreases with increase in temperature. In DMF, high values of ultrasonic velocity are observed. "However, in chloroform reverse order of compounds for ultrasonic velocity is obtained, indicating thereby that compound SB-2 containing 4-methyl group causes strong solute-solvent interactions in chloroform than 4-fluoro group (as in SB-1).
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
1. T. Sumathi, U. Maheswari, Ultrasonic and theoretical studies of some ternary liquid mixtures at various temperatures, Ind. J. Pure Appl. Physics, 47, 782-786 (2009). [ Links ]
2. J.M. Thijssen, The history of ultrasound techniques in ophthalmology, Ultrasound Med. Biol., 19, 599-618 (1993). [ Links ]
3. C.L. De Korte, M. Nillesen, A. Saris, R. Lopata, J. Thijssen, L. Kapusta, New developments in paediatric cardiac functional ultrasound imaging, J. Med. Ultrasonics, 41, 279-290 (2014). [ Links ]
4. B.J. Staples, B.L. Roeder, G.A. Husseini, O. Badamjav, G.B Schaalje, W.G. Pitt, Role of frequency and mechanical index in ultrasonic enhanced chemotherapy in rats, Cancer Chemother. Pharmacol., 64, 593-600 (2009). [ Links ]
5. R. Paproski, A. Forbrich, M. Hitt, R. Zemp, RNA biomarker release with ultrasound and phase-change nanodroplets, Ultrasound Med. Biol., 40, 1847-1856 (2014). [ Links ]
6. W.P. Mason, Sonics and ultrasonics: Early history and applications, IEEE Trans Son. Ultrasonic, 23, 224 (1976). [ Links ]
7. G. Gooberman, "Ultrasonics-Theory and application", The English University Press Ltd., London EC4, 1968. [ Links ]
8. V. Kühnel, U. Kaatze, Uncommon ultrasonic absorption spectra of "tetra alkyl ammonium bromides" in aqueous solution, J. Phys. Chem., 100, 19747-19757 (1996). [ Links ]
9. M.R. Doosti, R. Kargar, M.H. Sayadi, Water treatment using ultrasonic assistance: A review, Proc. Int. Acad. Ecology. Environ. Sci., 2, 96-110 (2012). [ Links ]
10. H.S. Lillard, Decontamination of poultry skin by sonication, Food Tech., 48, 72-73 (1994). [ Links ]
11. Z. Dolatowski, J. Stadnik, D. Stasiak, Applications of ultrasound in food technology, Acta Scientiarum Polonorum, Technologia Alimentaria, 6, 89-99 (2007). [ Links ]
12. S. Songül, C. Ysal, Use of ultrasound in food preservation, Nat. Sci., 5, 5-13 (2013). [ Links ]
13. R. Palani, A. Geetha, S. Saravanan, S. Tontapur, Physico-chemical behavior of binary liquid mixtures of some monohydroxy alcohols with DMSO as common solvent, Rasayan J. Chem., 1, 481-488 (2008). [ Links ]
14. A. Nain, D. Chand, Volumetric, ultrasonic and viscometric behavior of glycine, DL-alanine and L-valine in aqueous 1,4-butanediol solutions at different temperatures, J. Chem. Thermodyn., 41, 243-249 (2009). [ Links ]
15. M. Gowrisankar, P. Venkateswarlu, K. Sivakumar, S, Sivarambabu, Ultrasonic studies on molecular interactions in binary mixtures of N-methyl aniline with methyl isobutylketone + 3-pentanone, and + cycloalkanones at 303.15 K, J. Solution Chem., 42, 916-935 (2013). [ Links ]
16. R. Palani, A. Geetha, Acoustical and excess thermodynamic studies of molecular interaction in aqueous mixed solvent systems at 303, 308 and 313 K, Phys. Chem. Liq., 47, 542-552 (2009). [ Links ]
17. X. Wu, R.T. Edward, L. Kostetski, N. Kocherginsky, A.L.C. Tan, P. Wilairat, M.L. Go, Antiplasmodial activity of ferrocenyl chalcones: Investigations into the role offerrocene, Eur. J. Pharm. Sci., 27, 175-187 (2006). [ Links ]
18. B. Das, G. Mariappan, S. Saha, D. Bhowmik, Chiranjib, Anthelmintic and antimicrobial activity of some novel chalcone derivatives, J. Chem. Pharm. Res., 2, 113-112 (2010). [ Links ]
19. T. Suwa, K. Fukushima, K. Kyogoku, Effect of an anti-ulcer agent, 2'- carboxy-methoxy-4,4'-bis(3-methyl-2-butenyloxy) chalcone (SU-88), on the biosynthesis of gastric sulfated mucosubstances in restrained and water-immersed rats, Jap. J. Pharm., 34, 89-94 (1984). [ Links ]
20. K. Mallikarjun, Antiviral activity of substituted chalcones and their respective Cu(ii), Ni(ii) and Zn(ii) complexes, J. Chem., 2, 58-61 (2005). [ Links ]
21. J. Jeon, S. Kim, C. Kim, J. Kim, J. Jun, Synthesis of biologically active chalcones and their anti-inflammatory effects, Bull. Korean Chem. Soc., 33, 953-957 (2012). [ Links ]
22. O. Muraoka, T. Sawada, E. Morimoto, G. Tanabe, Chalcones as synthetic intermediates. A facile route to (±)-magnosalicin, an antiallergyneolignan, Chem. Pharm. Bull., 41, 772-774 (1993). [ Links ]
23. S. Gafner, J. Wolfender, S. Mavi, K. Hostettmann, Antifungal and antibacterial chalcones from Myricaserrata, Planta Medica, 62(1), 67-90 (1996). [ Links ]
24. S. Syam, S. Abdelwahab, M. Al-Mamary, S. Mohan, Synthesis of chalcones with anticancer activities, Molecules, 17, 617961-617995 (2012). [ Links ]
25. Y. Rajendra Prasad, A. Srinivasa-Rao, R. Rambabu, Synthesis of some 4'-amino chalcones and their antiinflammatory and antimicrobial activity, Asian J. Chem., 21, 907-914 (2009). [ Links ]
26. V. Mudalir, V. Joshi, Synthesis and insecticidal activity of new substituted phenoxychalocones, Ind. J. Chem., 34B, 456-457 (1995). [ Links ]
27. E. Oganesyan, A. Saraf, A. Simonyan, I. Shriyaev, Structure-activity relationship in flavonoids. Anti-allergic activity of chalcones, Pharm. Chem. J., 25, 526-530 (1991). [ Links ]
28. J. Riddick, W. Bunger, T. Sakano, "Organic solvents-physical properties and methods of purification Techniques of Chemistry", Wiley-Interscience Publication, John Wiley, New York, 1986. [ Links ]
29. G. Sastry, V. Satry, B. Krishnamurty, Ultrasonic parameters in mixed salt solutions, Ind. J. Pure Appl. Physics, 6, 637-638 (1986). [ Links ]
30. B. Jacobson, A. Anderson, J. Arnold, A Proton magnetic resonance study of the hydration of deoxyribonucleic acid, Nature (London), 173, 772-773 (1954). [ Links ]
31. S. Bagchi, S.K. Nema, R.P. Sing, Ultrasonic and rheological investigations of solid propellant binders, Eur. Polym. J., 25, 441-444 (1989). [ Links ]
32. C.V. Suryanarayana, J. Kuppuswamy, Role of internal pressure in the chemistry of electrolyte solutions, J. Acoust. Soc., 9, 4-8 (1981). [ Links ]
How to cite this article
Sh. Baluja, D. Lava, Ultrasonic behavior of various chalcones in some solvents at different temperatures, Rev. Colomb. Cienc. Quím. Farm., 45(3), 339-361 (2017).