INTRODUCTION
The infections causes by various microbes are dramatically increased during recent years [1]. Further, bacteria are becoming resistant to antimicrobial agents [2] so the effect of antimicrobial drugs available in the market is somewhat in doubt in future. These available antimicrobial drugs also have several drawbacks such asside effects, toxicity, low effectiveness and environmental issues [3,4]. Therefore, there is always need to develop new antimicrobials drugs for the treatment of infectious diseases [5].
Nitrogen and oxygen containing heterocyclic compounds like pyridine, coumarin etc., are always an attraction for researchers because of its efficiency towards various pharmacological usages [6,7]. Literature survey shows that large array of coumarin derivatives possess a variety of biological activities such as antihistaminic [8], anticancer [9], anti-fungal [10], analgesic [11], anti-tubercular [12], antioxidant [13], antimicrobial [14], anti HIV [15], etc. They are also used as herbicides [16], neuroimaging agent [17], fluorescent whitening agent [18], organic sensitizers in dye sensitized solar cells [19], etc.
Owing to these interesting applications of pyridyl-coumarine derivatives, in the present work, some new pyridyl-coumarinecompoundshave been synthesized. The structure of these compounds was confirmed by different spectroscopic techniques. Further, in vitro screening of these compounds was carried out against bacterial as well as fungal strains in N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO).
EXPERIMENTAL AND MATERIALS
Materials
The solvents, DMF (LOBA Chemie Pvt. Ltd. & CAS No.- 68-12-2) and DMSO (LOBA Chemie Pvt. Ltd. & CAS No.- 67-68-5) used for the study of antimicrobial activity were of Analytical Reagent (AR) grade supplied by LOBA Chemie Pvt. Ltd. (Mumbai-INDIA) and were purified according to the standard reported procedure [20].
Synthesis
Equimolar mixture of different substituted benzaldehydes (Spectrochem Pvt. Ltd. & CAS No-100-52-7) and malanonitrile (LOBA Chemie Pvt. Ltd. & CAS No.- 109-77-3) in methanol (Allied Chemical Chemie Pvt. Ltd. & CAS No.- 67-56-1) was stirred at room temperature (RT) in presence of catalytic amount of piperidine (Sigma Aldrich & CAS No.- 110-89-4). The reaction progress was checked by analytical thin layer chromatography (TLC) (Performed on aluminum coated plates Gel 60F254 (E. Merck)) using (0.5:0.5 v/v-hexane: ethyl acetate) as mobile phase. After completion of reaction, the obtained solid was filtered, washed with cold methanol and was dried under vacuum. The obtained crude product was used in next step without further purification.
Equimolar mixture of 2-benzylidenemalanonitrile derivatives (Int-1), 3-acetylcouma-rin and thiophenol (Sigma Aldrich & CAS No.- 108-98-5) in ethanol was refluxed in presence of tri ethylamine (TEA) (Sigma Aldrich & CAS No.- 121-44-8) used as a catalyst. The progress of reaction was checked by TLC using (0.9: 0.1 v/v-chloroform: methanol) as a mobile phase. After completion of reaction, the temperature of reaction mass was allowed to decrease up to room temperature. The obtained solid was separated by filtration, washed with cold methanol and dried.
The reaction scheme is given in Figure 1. Following five pyridyl-coumarin derivatives were synthesized.
QMS-1: 4-(4-fluorophenyl)-6-(2-oxo-2H-chromen-3-yl)-2-(phenylsulfanyl)pyridine-3-carbo nitrile
QMS-2: 4-(4-bromophenyl)-6-(2-oxo-2H-chromen-3-yl)-2-(phenylsulfanyl)pyridine-3-carbo nitrile
QMS-3: 4-(2-chlorophenyl)-6-(2-oxo-2H -chromen-3-yl)-2-(phenylsulfanyl)pyridine-3-carbo nitrile
QMS-4: 4-(4-(dimethylamino)phenyl)-6-(2-oxo-2H -chromen-3-yl)-2-(phenylsulfanyl) pyridine -3-carbonitrile
QMS-5: 4-(2-hydroxyphenyl)-6-(2-oxo-2//-chromen-3-yl)-2-(phenylsulfanyl)pyri-dine-3-carbo nitrile
All the synthesized pyridyl-coumarin derivatives were crystallized from ethanol before use. The purity of these synthesized compounds was checked by GC-MS (SHI-MADZU Model-QP2010) and was found to be greater than 99.98 %.
Spectroscopy study
The structure of the synthesized compounds was confirmed by FT-IR, 1H NMR, 13C NMR and mass spectral data. The IR spectra were taken on Fourier Transform InfraRed Spectrophotometer (SHIMADZU Model-IRaffinity-1S). 1H NMR and 13C NMR spectra were recorded on a Bruker AVANCE III at 400 MHzfrequency. In all the cases, NMR spectra were obtained in deuterated dimethyl sulfoxide (DMSO-d6) and in presence of tetra methyl silane used as an internal standard. The NMR signals are reported in δ ppm. Mass spectra were determined using direct inlet probe on a GC-MS (SHI-MADZU Model-QP2010) mass spectrometer.
Figures 2 to 5 show FT-IR, 1H NMR, 13C NMR and mass spectra respectively for QMS-1.
The melting points of compounds were measured by Differential Scanning Calorimeter (SHIMADZU Model-DSC-60) under nitrogen atmosphere (flow rate 100 ml/ min) and at 10 °C/min heating rate.
Microorganisms tested
The studied microorganisms were obtained from National Chemical Laboratory, Pune, India and were maintained at 4°C. The selected Gram positive bacteria for the present study were Bacillus cereus ATCC11778 (BC), Corynebacterium rubrum ATCC14898 (CR), Bacillus subtilis ATCC6633 (BS) and Staphylococcus aureus ATCC29737 (SA). The Gram negative bacteria were Klebsiella pneumoniae NCIM2719(KP), Staphylo-coccus typhimurium ATCC23564 (ST), Escherichia coli NCIM2931 (EC), Pseudomo-nas aeruginosa ATCC9027 (PA). The selected fungal strains were Candida albicans ATCC2091 (CA), Candidaglabrata NCIM3448 (CG), Candida epicola NCIM3367 (CE) and Cryptococcus neoformans NCIM3542 (CN).
The agar well diffusion method [21] was used to study in vitro antimicrobial study of the synthesized compounds.For each compound in each solvent for a particular strain, the experiment was repeated three times. The average of these three values is graphically represented in Figures 6 to 8 along with uncertainty values.
RESULTS AND DISCUSSION
Table 1 shows the physical constant of synthesized compounds along with their side chain substitutions.
Spectral data
QMS-1:
IR (cm -1 ): 3394.72, 3317.56, 3224.98 (-OH stretching, H-bonded and/or -NH-stretching), 2934.67 (-CH- stretching), 2214.28 (-CN stretching), 1639.49, 1608.63 (C=Ostretching), 1523.76 (-CH- bending), 1346.31 (-CH- rock), 1261.45, 1230.58, 1203.58 (C-O stretching), 1099.43 (C-O stretching), 1026.13 (C-N stretching), 955.27, 806.25, 786.96 (substituted benzene).
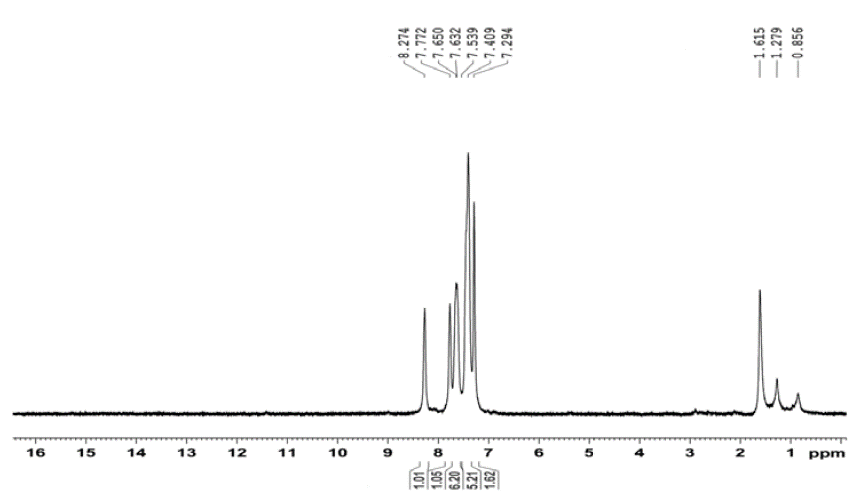
1 H NMR (DMSO-d 6 , 400 MHz) (δ ppm): 7.2942-7.3183 (2H, doublet, -CH- aromatic, J= 9.64 Hz), 7409-7.539 (5H, multiplet, -CH- aromatic), 7.6324-7.6550 (6H, multiplet, -CH- aromatic), 7.7726 (1H, singlet, -CH- aromatic), 8.2743 (1H, -CH- aromatic).
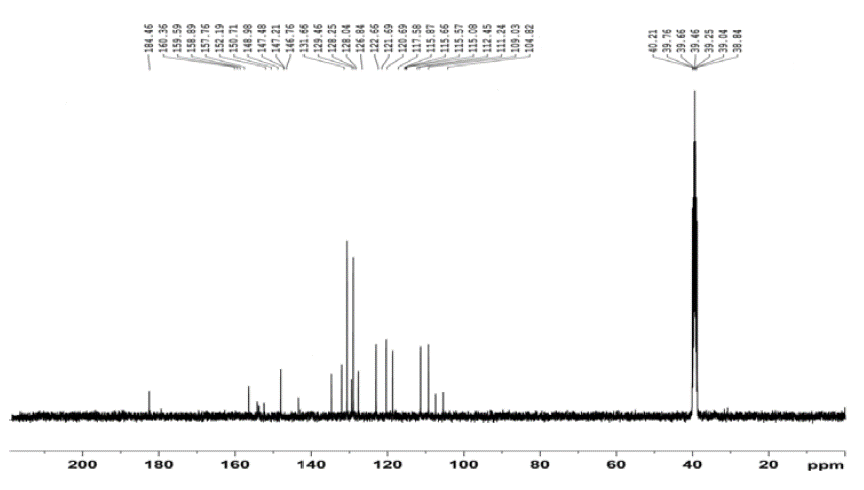
13 CNMR (DMSO-d 6 , 400 MHz) (δ ppm): 104.82, 109.03, 111.24, 112.45, 115.08, 115.57, 115.66, 115.87, 117.58, 120.69, 121.69, 122.66, 126.84, 128.04, 128.25, 129.40, 131.66, 146.76, 147.21, 147.48, 148.98, 150.71, 152.59, 160.36, 184.46.
Mass (m/=): 450.
QMS-2:
IR (cm 1 ): 3352.67, 3339.37, 3228.29 (-OH stretching, H-bonded and/or -NH-stretching), 2978.09, 2885.51 (C-H stretching alkane), 2299.15, 2260.57, 2214.28 (-CN stretching), 1643.55 (C=Ostretching), 1592.61, 1481.33, 1404.18 (-CH-bending), 1369.46, 1346.31, 1327.03 (-CH- rock),1261.45, 1203.58 (C-O stretching), 1138.00, 1118.71, 1099.43 (C-C stretching), 1026.13 (C-N stretching),752.24, 671.23 (substituted benzene).
1 H NMR (DMSO-d 6 , 400 MHz) (δppm): 6.9637-6.9783 (2H, doublet, -CH- aromatic,), 7.3734-7.5493 (3H, multiplet, -CH- aromatic), 7.5538-7.6132 (2H, doublet, -CH - aromatic), 7.6263-7.7743 (6H, multiplet -CH- aromatic), 8.0958 (1H, singlet, -CH-aromatic), 8.2769 (1H, singlet, -CH- aromatic).
13 CNMR (DMSO-d 6 , 400 MHz) (δ ppm): 104.38, 109.63, 113.68, 112.96, 115.21, 115.54, 115.98, 127.36, 120.48, 121.13, 122.67, 126.38, 128.09, 129.48, 146.28, 147.79, 147.73, 148.29, 150.68, 153.26, 157.69, 158.26, 159.18, 160.09, 185.56.
Mass (m/(): 511.
QMS-3:
IR (cm 1 ): 3356.28, 3356.09, 3223.67 (-OH stretching, H-bonded and/or -NH-stretching), 2981.95, 2881.65 (C-H stretching alkane), 2349.30, 2299.15, 2214.28 (-CN stretching), 1631.78, 1604.77 (C=Ostretching), 1550.77, 1523.76, 1504.48 (n-H bending), 1481.33, 1442.75 (-CH- bending), 1369.46 (-CH- rock), 1296.16, 1222.87 (C-O stretching), 1157.29, 1099.43, 1026.13 (C-N stretching),779.24, 686.66 (substituted benzene).
1 HNMR (DMSO-d6, 400 MHz) (δ ppm): 7.2942-7.3372 (2H, doublet, -CH- aromatic), 7.4932-7.3721 (5H, multiplet, -CH- aromatic), 7.6348-7.6529 (6H, multiplet, -CH- aromatic), 7.2834 (1H, singlet, -CH- aromatic), 8.2758 (1H, -CH- aromatic).
13 CNMR (DMSO-d 6 , 400 MHz) (δ ppm): 104.48, 109.49, 113.29, 112.60, 115.02, 115.35, 115.89, 127.39, 120.67, 121.59, 122.27, 126.29, 127.46, 128.27, 129.58, 146.37, 147.68, 147.28, 148.48, 150.79, 153.26, 157.47, 158.79, 159.39, 160.29, 185.49.
Mass (m/( ): 466. QMS-4:
IR (cm 1 ): 3378.98, 3312.67, 3221.45 (-OH stretching, H-bonded and/or -NH-stretching), 2978.09 (C-H stretching alkane), 2349.30, 2299.15, 2210.42 (-CN stretching), 1708.93, 1639.49 (C=Ostretching), 1546.91, 1523.76, 1442.75 (-CH- bending), 1273.02, 1203.58, 1118.71, 1099.43 (C-O stretching), 1080.14, 1026.13 (C-N stretching), 756.10, 686.66, 671.23 (substituted benzene).
1 HNMR (DMSO-d 6 , 400 MHz) (δppm): 3.4657 (3H, singlet -CH3), 3.5783 (3H, singlet -CH3), 7.2958-7.3349 (2H, doublet, -CH- aromatic), 7.4939-7.3749 (5H, multiplet, -CH- aromatic), 7.6359-7.6527 (6H, multiplet, -CH- aromatic), 7.2583 (1H, singlet, -CH- aromatic), 8.2402 (1H, -CH- aromatic).
13 C NMR (DMSO-d 6 , 400 MHz) (δ ppm): 23.67, 24.38,104.84, 109.63, 113.28, 112.47, 115.02, 115.35, 115.78, 127.36, 120.48, 121.59, 122.62, 126.29, 128.27, 129.90, 146.37, 147.68, 147.28, 148.69, 150.39, 153.28, 157.90, 158.38, 159.18, 160.29, 185.49.
Mass (m/( ): 475.
QMS-5:
IR (cm 1 ): 3378.65, 3324.56, 3222.78 (-OH stretching, H-bonded and/or -NH-stretching), 2978.09, 2885.51 (C-H stretching alkane), 2384.02, 2349.30, 2299.15, 2214.28 (-CN stretching), 1708.93 (C=Ostretching), 1512.19 (N-H bending), 1442.75 (-CH- bending), 1369.46, 1346.31 (-CH- rock), 1273.02, 1234.44, 1199.72 (C-O stretching), 1118.71, 1068.56, 1026.13 (C-N stretching), 763.81, 740.67, 682.80 (substituted benzene).
1 HNMR (DMSO-d6, 400 MHz) (δ ppm): 7.2948-7.3338 (2H, doublet, -CH- aromatic), 7.4958-7.3782 (5H, multiplet, -CH- aromatic), 7.6359-7.6924 (6H, multiplet, -CH- aromatic), 7.2548 (1H, singlet, -CH- aromatic), 8.2423 (1H, -CH- aromatic).
13 CNMR (DMSO-d 6 , 400 MHz) (δppm): 104.58, 109.39, 113.68, 112.29, 115.89, 115.37, 115.69, 127.39, 120.97, 121.58, 122.69, 126.38, 127.36, 128.79, 129.90, 136.78, 146.36, 147.48, 147.68, 148.12, 150.45, 153.78, 157.90, 158.23, 159.56, 160.78, 185.12.
Mass (m/(): 448.
IR spectra
The IR spectrum of QMS-1 is given in Figure 2. The peaks observed around 32003500 cm-1 are due to stretching of -OH (H-bounded) and/or -NH- groups. The peak around 2929-2978 cm-1 is of -CH stretching of aromatic ring. The-CN stretching is observed around 2300-2200 cm-1. The peaks for -C=O and C-H stretching are obtained around 1600-1700 cm-1 and 1550-1600 cm-1 respectively whereas alkane C-H bending peak is observed around 1469-1490 cm-1. The peaks observed around 1300-1334 cm-1 are due to C-O stretching of ester group and/or ether group. The -CN stretching is observed around 1250-1050 cm-1.
1 HNMR spectra
The 1H NMR spectrum of QMS-1 is shown in Figure 3. For aromatic protons, peaks are between 7.2940 to 7.6500 δ ppm with their appropriate multiplicity. Two singlet peaks of aromatic proton (=CH-) are observed at 7.7120 and 8.2740.
All the 1H NMR peaks suggests that compounds are synthesized successfully.
13 CNMR spectra
Figure 4 shows the 13C NMR spectrum of compound QMS-1. The aromatic carbons of phenyl rings are shown between 104.82 to 184.46 δ ppm with their appropriate multiplicity.
Mass spectra
Figure 5 shows the mass spectrum of compound QMS-1. From mass fragmentation, the structures of synthesized compounds are confirmed.
Antimicrobial activity
Figure 6 shows the zone of inhibition for the studied compounds against Gram positive bacteria in DMF and DMSO along with two standard antibiotics. It is observed that against Bacillus cereus, all the studied compounds exhibited inhibition (except QMS-4 in DMSO) in both DMF and DMSO. However, in DMF, QMS-5 showed maximum inhibition and this value is higher than tetracyclin but lower than Chloramphenicol.
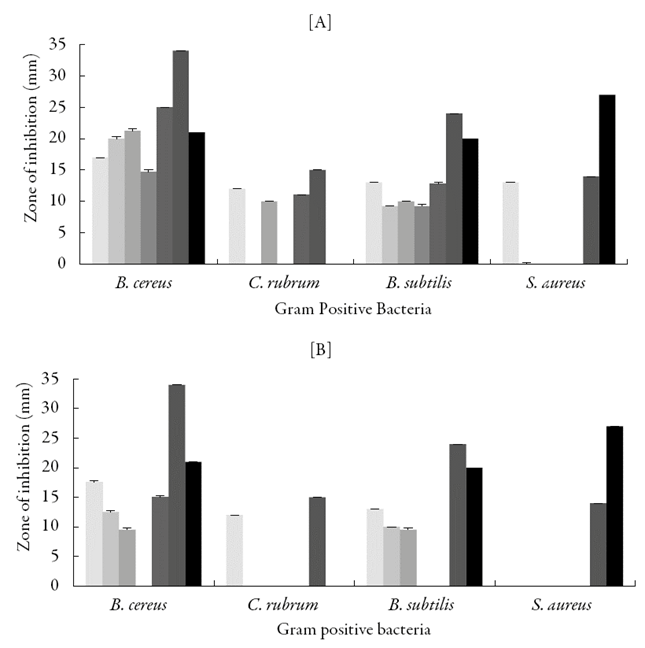
Figure 6 Antibacterial activity of synthesized compounds against Gram positive bacteria in [A] DMF and [B] DMSO. [QMS-1, ( ); QMS-2, (
); QMS-2, ( ); QMS-3, (
); QMS-3, ( ); QMS-4, (
); QMS-4, ( ); QMS-5, (
); QMS-5, ( ); Chloram-phenicol (
); Chloram-phenicol ( ); Tetracyclin (
); Tetracyclin ( )].
)].
However in DMSO, QMS-1 showed maximum inhibition against Bacillus cereus but lower than both the antibiotics.
In DMF, QMS-1, QMS-3 and QMS-5 showed inhibition against Corynebacterium rubrum whereas in DMSO only QMS-1 was found to be effective against this bacterial strain. Against Bacillus subtilis in DMF, all the studied compounds exhibited significant inhibition. However in DMSO, QMS-1, QMS-2 and QMS-3 exhibited inhibition. In DMF, against Staphylococcus aureus only QMS-1 showed inhibition whereas in DMSO, none of compounds was found to be effective.
This suggests that inhibition depends on solvent, structure of compound and bacterial strain. In the present work, all the studied compounds have the same central moiety but different substitution groups as listed in Table 1. QMS-5 contains 2-hydroxy group which shows maximum inhibition against Bacillus cereus in DMF than other substitutions. However in DMSO, 4-fluoro (as in QMS-1) group showed significant inhibition against this bacterial strain. In DMF, against Bacillus subtilis and Staphylococcus aureus, again 4-fluoro group (as in QMS-1) is most effective. However, in DMF, against Bacillus subtilis, 4-bromo (as in QMS-2) and 4-N,N-dimethylamine (as in QMS-4) groups are also found to be effective almost up to same extent. None of the groups are found to be effective against Staphylococcus aureus in DMF and DMSO except QMS-1 containing 4-fluoro group in DMF.
Thus, for the studied compounds, DMF is better solvent against selected Gram positive bacteria.
Figure 7 shows the zone of inhibition against Gram negative bacteria in both DMF and DMSO. In DMF, against Klebsiella pneumoniae, compounds QMS-1 containing 4-fluoro, QMS-4 containing 4-VJVT-dimethylamine and QMS-5 containing 2-hydroxy groups exhibited significant inhibition then antibiotic chloramphenicol and inhibition is maximum for QMS-1. In DMSO, compounds QMS-1, QMS-2 and QMS-3 showed significant inhibition against Klebsiella pneumonia and inhibition of QMS-1 is almost to the same extent as chloramphenicol. Thus, in DMF and DMSO, 4-fluoro group is found to be most effective against Klebsiella pneumonia.

Figure 7 Antibacterial activity of synthesized compounds against Gram negative bacteria in [A] DMF and [B] DMSO. [QMS-1, ( ); QMS-2, (
); QMS-2, ( ); QMS-3, (
); QMS-3, ( ); QMS-4, (
); QMS-4, ( ); QMS-5, (
); QMS-5, ( ); Chloram-phenicol (
); Chloram-phenicol ( ); Tetracyclin (
); Tetracyclin ( )].
)].
Against Staphylococcus typhimurium, only QMS-1 and QMS-5 having 4-fluoro and 2-hydroxy groups respectively showed inhibition in DMF whereas in DMSO except QMS-2, other compounds exhibited inhibition. Thus, in DMSO 4-bromo group is not effective against this strain. Against Escherichia coli in DMF, only QMS-1 showed inhibition whereas in DMSO, compounds QMS-3 and QMS-5 containing 2-chloro and 2-hydroxy groups respectively showed inhibition. Against Pseudomonas aeruginosa, none of the studied compounds are effective in DMF whereas in DMSO, only 4-bromo (as in QMS-2) group showed inhibition and up to same extent with tetracyclin.
Hence, the synthesized compounds showed better activity in DMSO against Gram negative bacteria.
Figure 8 shows the zone of inhibition for the studied compounds and two antibiotics such as nystatin and itroconazolagainst selected fungal strain in DMF and DMSO. Against Candida albicans, in DMF except QMS-3, other compounds exhibited significant inhibition and QMS-5 containing 2-hydroxy group showed maximum inhibition. However, in DMSO none of the studied compounds are found to be effective against this fungal strain. In DMF, none of compound was found to inhibit Candida glabrata and Candida epicola. Whereas in DMSO, only QMS-3 showed some inhibitionagainst Candida glabrata. Against Candida epicola, there was no inhibition by any of the compound in DMSO. Against Cryptococcus neoformans in DMF, only QMS-1 having 4-fluoro group showed inhibition whereas in DMSO, none of the studied compounds was effective.
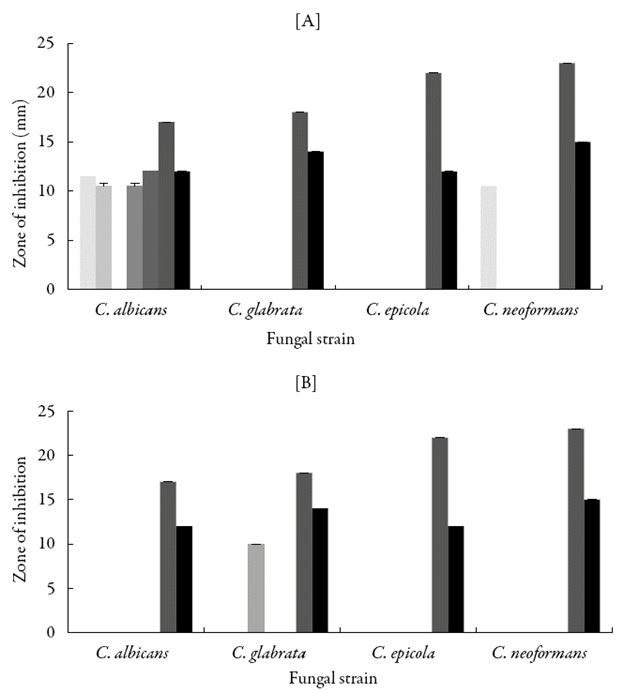
Figure 8 Antifungal activity of synthesized compounds in [A] DMF and [B] DMSO. [QMS-1, ( ); QMS-2, (
); QMS-2, ( ); QMS-3, (
); QMS-3, ( ); QMS-4, (
); QMS-4, ( ); QMS-5, (
); QMS-5, ( ); Nystatin (
); Nystatin ( ); Itroconazol (
); Itroconazol ( )].
)].
Overall, Staphylococcus aureus, Candida albicans, Candida epicola and Cryptococcus neoformans are most resistant strains.
CONCLUSIONS
The inhibition against bacterial and fungal strains depends upon the solvent, structures of compound and strain. For the selected Gram positive bacterial and fungal strains, DMF is better solvent whereas for Gram negative bacteria, DMSO is better solvent. Compounds having halogen groups are more effective against selected microbial strains. Staphylococcus aureus, Candida albicans, Candida epicola and Cryptococcus neoformans are the most resistant strains.