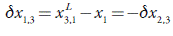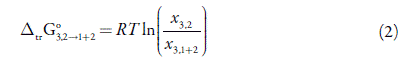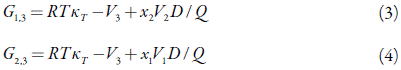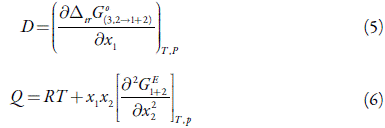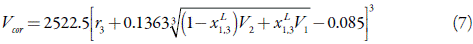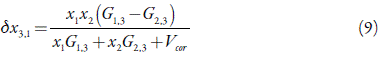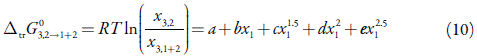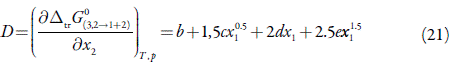INTRODUCTION
Tricin (5,7-dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)chromen-4-one) (Figure 1) is a flavone often found in the outer layer of cereal crops, has also been associated with reduction in cancer. In a mouse model of intestinal cancer, addition of tricin to the diet (0.2%, which was about 6 mg per mouse per day) resulted in 33% fewer intestinal adenomas [1]. In another study, dietary tricin was found to inhibit inflammation-induced colon cancer in mice [2]. Additional beneficial health effects of tricin are summarized in a recent review [3].
In foods tricin is mainly found in whole cereal grains, such as rice, barley, oat, and wheat, also, in medicinal plants such as Artemisia copa [4]. However, higher levels of tricin and its derivatives are often found in other nonedible tissues, such as leaves and grain hulls. Because of the potential therapeutic use of tricin and its derivatives, extraction from such nonedible sources has been proposed [5]. Tricin has also recently been found as a component of lignin in some species, particularly monocots [6].
Within the context of this article, the studies of the solubility of drugs in solvent mixtures have as their purpose the modeling of the solubilities, with the aim of reducing the number of experimental tests, and in the best case, to bring them to zero [7-12]. Cheng et al. [13] determined the solubility of Tricin in {ethanol (1) + water (2)} cosolvent mixtures at diferent temperatures. Some thermodynamic functions associated to the dissolution of this drug were obtained, but nothing about the drug is preferentially solvated by water and by ethanol in the aqueous solvent mixtures. Nevertheless, more insight into the interactions of the drug molecules with those of the components of the mixed solvent would help in the choice of better co-solvents for the enhancement of drug solubilities in aqueous media [14-16].
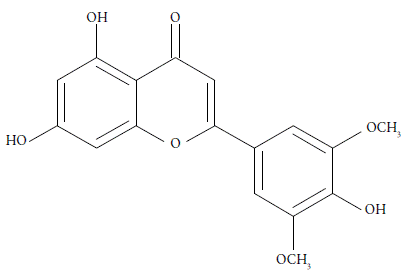
The professor Marcus addressed this problem by means the inverse Kirkwood-Buff integral (IKBI) method [17,18]. Where the results are expressed in terms of the preferential solvation parameter δx3,1 for the solute tricin (3) by the component solvents ethanol (1) and water 82):
where (δx3,1 is the local mole fraction of 1 in the surroundings of the solute 3 and x1 is its mole fraction in the bulk solvent mixture. 3 is preferentially solvated by 1 when (δx3,1> 0, otherwise by 2. Negligible preferential solvation is indicated when | (δx3,1| ≤ 0.01, but values δx3,1 ≈ x 2 signify δx3,1 ≈ 1 or complete selective solvation of 3 by 1. The magnitude and shape of the (δx3,1= f (x 1) curve provide the desired information on the relative strength of the interactions of 3 with 1 and with 2 in their mixture.
In order to apply the IKBI method, the solubility data need to be transformed into standard molar Gibbs energies of transfer from one of the components, say 2, into the mixture 1+2. If x2 is the solubility as a function of the solvent composition, then:
Preferential solvation
The IKBI approach depends on obtaining the Kirkwood-Buff integrals as follows [17-22]
Here G 1,3 and G 2,3 are the Kirkwood-Buff integrals (in cm3 mol-1) as obtained from the thermodynamic data: the isothermal compressibility of the solvent mixtures, κ τ (in GPa-1), and standard partial molar volume of solute in this mixture, V3 (in cm3 mol-1). The functions.
D and Q (in kJ mol-1, as is RT) are given in equations (5) and (6), and depend on the first derivative of standard molar Gibbs energies of transfer, ∆tr G o, and the second derivative of the excess Gibbs energy of mixing of the two solvents, G E 1+2 , with respect to the composition [23,24].
A final quantity that is required is the correlation volume around 3, V œr , within which preferential solvation takes place and the local mole fraction, xL 1,3, is defined [18,25].
This volume involves one solvation shell and depends on the size of the solute molecule (its radius, r3 in nm, equation 8) plus a distance of one mean solvent diameter from the surface of the solute.
The resulting correlation volume, in cm3 mol-1, the numerical coefficients of which relate sizes to volumes, requires iteration, since it involves the local mole fractions of the two solvents.
Finally, the resulting preferential solvation parameter is then:
However, the correlation volume requires iteration, because it depends on the local mole fractions [19,26,27].
RESULTS AND DISCUSSION
The experimental solubility of tricin (3) in {ethanol (1) + water (2)} mixtures (Fig. 2) was taken from the literature [13].
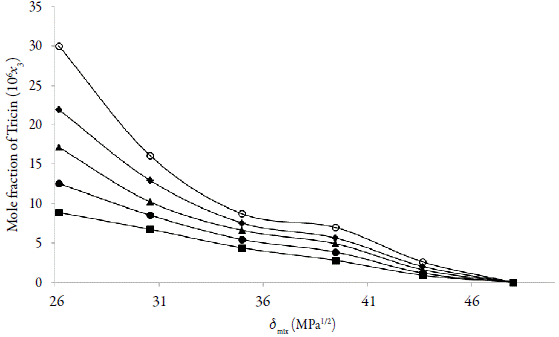
Figure 2 Experimental molar solubility of Tricin in {ethanol (1) + water (2)} mixtures at different temperatures; (■=293.15 K; ●=298.15 K; ▲=303.15 K; 4=308.15 K; ♦= 313.15 K). Data taken from Cheng et al. (2016) [6]. (106 x3).
The solubility increases with temperature in all cases indicating that the dissolution process is endothermic. The highest solubility of tricin (3) expressed as a mole fraction were obtained in near ethanol (1) at T = 313.15 K, whereas the lowest values were found in pure water (2) at 293.15 K (Figure 2).
On the other hand, Fig. 2 depict the solubility profiles as a function of the polarity of the mixtures, expressed by their solubility parameters (δ mix). For a binary mixture δ mix is calculated from the solubility parameters of the pure solvents (δ 1 = 26.5 MPa1/2 and δ 2 = 47.9 MPa1/2 [28,29]).
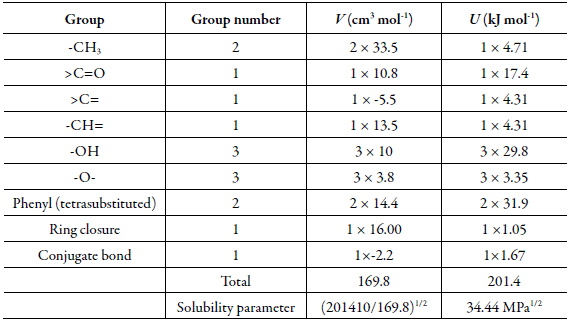
The solubility parameter of solute, estimated according to the groups contribution method proposed by Fedors [21], is δ 3 = 34.44 MPa1/2 (Table 1). Usually, the maximum solubility of a substance is found in a solvent or mixture of solvents with polarity similar to that of the solute. Tis behavior has been described by Delgado and Martinez in several investigations [30-37]. However, the experimental data did not present a maximum in a mixture but in pure ethanol (1).
Table 1 Application ofthe Fedors' method to estimate internal energy, molar volume, and Hildebrand solubility parameter of Tricin (3).
Preferential solvation
Standard molar Gibbs energy of transfer of tricin from neat water to {ethanol (1) + water (2)} mixtures is calculated and correlated to a non-regular polynomial from the drug solubility data by using equation (10). Figure 3 shows the Gibbs energy of transfer behavior at 323.15 K. Tus, the coefficients of the polynomials are shown in Table 2.
Table 2 Coefficients of the equation (10) applied to the Gibbs energy of transfer of tricin (3) from neat water (2) to {ethanol (1) + water (2)} mixtures at several temperatures.
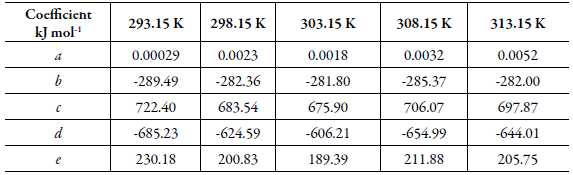
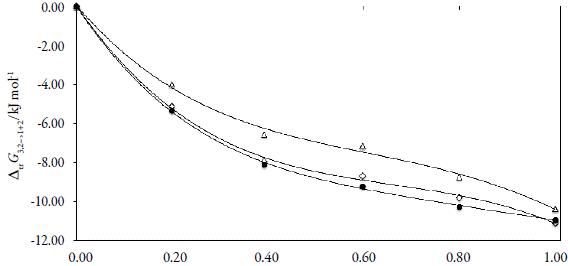
Figure 3 Gibbs energy of transfer of tricin (3) from neat water (2) to {ethanol (1) + water (2)} co-solvent mixtures at some temperatures (●=293.15 K; ◊=303.15 K; Δ=313.15 K).
Thus D values are calculated from the first derivative of polynomial models (Equation 11) solved according to the co-solvent mixtures composition. This procedure was done varying by 0.05 in mole fraction of methanol but in the following tables the respective values are reported varying only by 0.10.
Q and RT κ τ values for {ethanol (1) + water (2)} binary mixtures, as well as the partial molar volumes of ethanol (1) and water (2), at the three temperatures considered here, were taken from the literature [22,38,39].
Otherwise, partial molar volumes of nonelectrolyte drugs are not frequently reported in the literature. This is because of the large uncertainty obtained in its determination due to their low solubilities, in particular in aqueous media. For this reason, in the first approach, the molar volume of tricin was considered as independent of co-solvent composition and temperature, as they are calculated according to the groups contribution method proposed by Fedors [29]. On the other hand, the radius of the drug molecule was calculated by using equation 8, as r3 = 0.407 nm.
In order to apply the IKBI method, the correlation volume was iterated three times by using the equations (1), (7) and (9) to obtain the final values reported in Table 3. This property is almost independent on temperature in water-rich mixtures but increases to some extent in ethanol-rich mixtures. This would be expectable according to the variation of the respective molar expansibilities with the mixtures composition [40].
Table 3 Correlation volume (cm3 mol-1) for tricin in {ethanol (1) + water (2)} co-solvent mixtures at several temperatures after three iterations.
| X1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K |
|---|---|---|---|---|---|
| 0.00 | 790.74 | 791.26 | 791.60 | 792.09 | 792.87 |
| 0.10 | 814.11 | 810.66 | 808.86 | 812.33 | 813.15 |
| 0.20 | 938.40 | 939.33 | 941.50 | 944.50 | 946.85 |
| 0.30 | 1033.69 | 1036.60 | 1039.18 | 1041.04 | 1043.50 |
| 0.40 | 1115.76 | 1118.16 | 1119.75 | 1122.04 | 1124.45 |
| 0.50 | 1196.96 | 1197.75 | 1198.42 | 1202.63 | 1205.53 |
| 0.60 | 1281.26 | 1280.71 | 1281.36 | 1288.19 | 1292.30 |
| 0.70 | 1363.04 | 1364.41 | 1367.61 | 1376.55 | 1383.36 |
| 0.80 | 1428.86 | 1435.71 | 1443.43 | 1451.91 | 1462.05 |
| 0.90 | 1485.75 | 1493.95 | 1501.84 | 1507.52 | 1516.46 |
| 1.00 | 1560.82 | 1566.06 | 1571.22 | 1576.68 | 1582.68 |
The preferential solvation parameter, δx 1,3 for ethanol (1) around tricin (3) is displayed in Fig. 4 at five temperatures. The values of δx 13 vary non-linearly with the proportion of ethanol (1) in the alcoholic mixtures (figure 4). The addition of ethanol to water causes a negative change in δx 1,3 from pure water (2) up to the 0.20 in molar fraction of ethanol (1) reaching minimum values near to -0.08 at 0.05 in molar fraction of ethanol at 303.15 K. In this composition, water is preferred over ethanol around the tricin (3), this because possibly the structuring of water molecules around the non-polar groups of this drug leading to hydrophobic hydration of the aromatic and methyl groups (Fig.1), contributes to lowering of the net δx 1,3 to negative values in these water-rich mixtures. This behavior is observed at all study temperatures as can be seen in Table 4. Although, a clear influence of the temperature in water-rich mixtures is not perceived, in ethanol-rich mixtures the solvation by ethanol increases with the increase in temperature what matches the solubility profile.
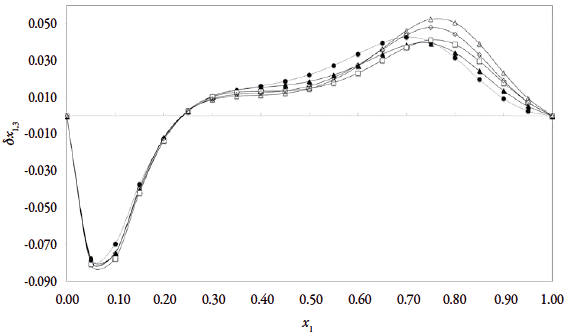
Figure 4 δx 1,3 values tricin (3) in {ethanol (1) + water (2)} co-solvent mixtures at several temperatures. (●=293.15 K; ▲=298.15 K; □=303.15 K; ◊=308.15 Δ; =313.15 K).
Table 4 δx1,3, values of tricin (3) in {ethanol (1) + water (2)} co-solvent mixtures at several temperatures.
| X 1 | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K |
|---|---|---|---|---|---|
| 0.00 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 0.10 | -0.070 | -0.075 | -0.078 | -0.075 | -0.075 |
| 0.20 | -0.012 | -0.013 | -0.013 | -0.012 | -0.012 |
| 0.30 | 0.010 | 0.011 | 0.010 | 0.009 | 0.009 |
| 0.40 | 0.016 | 0.015 | 0.013 | 0.012 | 0.011 |
| 0.50 | 0.022 | 0.019 | 0.015 | 0.016 | 0.015 |
| 0.60 | 0.034 | 0.027 | 0.023 | 0.028 | 0.027 |
| 0.70 | 0.043 | 0.038 | 0.037 | 0.044 | 0.046 |
| 0.80 | 0.032 | 0.034 | 0.039 | 0.044 | 0.051 |
| 0.90 | 0.009 | 0.014 | 0.018 | 0.019 | 0.023 |
| 1.00 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
In the mixtures with composition 0.25 < x1 < 1.00, the local mole fraction of ethanol is greater than the one in the bulk and it increases with the temperature increasing. In this way, the co-solvent action may be related to the breaking of the ordered structure of water (hydrogen bonds) around the nonpolar moieties of the drug which increases the solvation of the tricin and has a maximum value near to x1 = 0.75, i.e. (δx 1,3 = 0.052. On the other hand, although, as mentioned above, the maximum solubility was not achieved in a cosolvent mixture, whit would be expected theoretically, the maximum solvation is achieved in mixtures with polarities similar to those of tricin (3), this factor could also be involved in these behaviors.
The tricin (3) could act in solution as Lewis acids due to the hydrogen atom in their -OH groups in order to establish hydrogen bonds with proton-acceptor functional groups in EtOH and water (oxygen atom in -OH). In addition, these drugs could act as Lewis bases due to free electron pairs in oxygen atoms of hydroxyl and alkoxy groups to interact with hydrogen atoms present in both solvents. In this context, tricin has three hydrogen-bonding donor and seven hydrogen-bonding acceptor groups.
According to the preferential solvation results, it is conjecturable that in intermediate composition mixtures and ethanol-rich mixtures, tricin (3) is acting as Lewis acids with the ethanol molecules because this co-solvent is more basic than water as indicated by the Kamlet-Taft hydrogen bond acceptor parameters (β), i.e. 0.75 for ethanol (1) and 0.47 for water (2) [41,42]. In this way, tricin would prefer ethanol (1) instead of water. This behavior is similar to that presented by other drugs in aqueous cosolvent systems [27,43,44].
CONCLUSIONS
Explicit expressions for local mole fraction of ethanol and water around of tricin were derived on the basis of the IKBI method applied to equilibrium solubility values of this drug in {ethanol (1) + water (2)} mixtures. According to the performed analyses, tricin is preferentially solvated by water (2) in water-rich mixtures but preferentially solvated by ethanol (1) in mixtures with intermediate composition and those rich in ethanol at all temperatures considered.