INTRODUCTION
Cadmium (Cd) is a heavy metal belonging to class II-B of the periodic table [1] that, even at low concentrations, has toxic effects [2, 3]. In addition, Cd is a widely distributed pollutant in several sources as industrial waste, plastics and odontologic materials [2], cigarette smoke [4], forest fires [3], pigments [5], and fertilizers [6] and thus causes food and water contamination [7, 8].
Cd2+ bioaccumulates primarily in the liver, kidneys, bones, and pancreas [9, 10], which ultimately results in hepatic and renal dysfunctions, as well as osteoporosis and pancreatic cancer [11].
The mechanism by which Cd2+ induces cellular injuries appears to be supported, at least in part, by apoptotic pathways [3, 12]. Furthermore, the potential injury caused by Cd2+ appears to be associated with our own ability to induce protein denaturation and oxidative stress and decrease the enzymatic activity [13-15].
However, only a few studies have evaluated the genotoxicity in human cells exposed to lower concentrations of Cd. Herein, we report the in vitro minimal Cd2+ concentrations that could cause genotoxicity and cytotoxicity in human leukocytes. These screening experiments were performed at concentrations ranging from 1 to 25 (g/mL, which are significantly lower than those cited in the literature. We also determined the intracellular Cd2+ levels after cellular exposure to understand the relationship with the observed cell damage and also to elucidate the kinetic entrance of Cd2+ into human leukocytes under experimentally controlled conditions.
MATERIALS AND METHODS
Ethical aspects
The experimental protocols performed in this study, including the collection of venous blood samples, were submitted to and approved by the Universidade Federal de Santa Maria (RS, Brazil) Research Ethics Committee under the registration number 0089.0.243.000-07.
Material preparation
The study material was prepared as previously described [16]. Briefly, the material that was used for in vitro assays was new and disposable to avoid Cd2+ contamination. Control samples of the reagents were tested to identify metal contamination. Before use, all glassware was immersed in 20% aqueous HNO3 (v/v) for 24 h and subsequently rinsed with deionized water.
Both the comet and cell viability assays were performed in triplicate, while six replicates were analyzed to quantify the intracellular Cd2+content. The following screening strategy was performed for all assays: (1) negative control-adjusted leukocyte suspension (LS) (104 cells/mm3) in phosphate-buffered saline (PBS; pH 7.4); (2) positive control-LS plus 4 mM H2O2; tests-composed of LS with either 1, 2.5, 5, 10, 15, or 25 μg/mL Cd2+ (CdCl2 analytical grade, Merck®, Darmstadt, Germany). This concentration range was chosen based on specialized literature data that related the metal concentration with cellular or DNA damage [17-19]. All sample groups were incubated for 1 h at room temperature (25°C) under slow stirring of which the direction was inverted every 10 min. Thereafter, the abovementioned tests were performed, as described below, with the exception of the intracellular Cd2+quantification. In this case, the samples were additionally washed five times with PBS (pH 7.4) and centrifuged at 2000 rpm for 10 min at room temperature.
Intracellular Cd2+ Content (Inductively Coupled Plasma Mass Spectrometry-ICP-MS)
The intracellular Cd2+ concentration in the leukocytes was quantified using ICP-MS analysis. The samples were adequately diluted in 5% HNO3 (v/v) by the factor required to obtain within the concentration range of the calibration curve and analyzed by ICP-MS (PerkinElmer SCIEX, Model Elan DRC II, Thornhill, Canada) to determine the extracted Cd content. A certified reference material was used for the measurements, which resulted in consistent concentrations (98%-101%). Special care was taken concerning the material and the treatment of the samples (human leukocytes).
The water used was previously distilled and deionized in an ion-exchange column and purified using a Milli-Q system (Millipore ®, Billerica, USA) with a resistivity of 18.2 MO/cm. The reagent used for the decomposition of LS (HNO3, 65%, 1.4 kg/L, Merck®, Darmstadt, Germany) was of redistilled analytical grade (model duoPUR distillation Subboiling Distillation System, Milestone ®, Sorisole, Italy).
Argon (99.999 % purity, White Martins, Brazil) was used for plasma generation. Hydrochloric acid solution was prepared by diluting the concentrated acid in water. Sodium tetrahydroborate (NaBH4, PA, minimum purity of 97%, Nuclear®, Brazil) solution was prepared by dissolving the solid reagent in a 0.1% (w/v) NaOH solution (minimum purity of 99%, Vetec ®, Brazil). Cd2+ was added from a stock solution of CdCl2 (1000 mg/L). All materials were decontaminated through immersion in an aqueous HNO3 20% (v/v) solution for 24 h and subsequently rinsed with distilled/ deionized water.
The leukocyte decomposition was done by the wet method in an open system (block digester-Tecnal, model TE 040/25 for 40 quartz tubes, aluminum body, and thermostat). The samples were weighed and transferred to glass tubes (2.5 x 40 cm), followed by addition of 4 mL of concentrated HNO3 and 1 mL of 1 N HCl. The temperature was increased to 50 °C for 20 min and 90°C for 4 h and 30 min. Glass funnels were placed on the upper end of the pipes for the efficiency of the digestion procedure. After cooling to room temperature, the solutions were transferred to graduated polypropylene vials, and the volume was adjusted to 25 mL using ultra-purified water. The samples were adequately diluted in 5% HNO3 (v/v) by the factor required to obtain within the concentration range of the calibration curve and analyzed by ICP-MS.
The intracellular Cd2+ content was calculated, considering the number of 104 cells/ mm3 from LS, taking into account the Cd2+ concentration to which the cells were exposed and the value determined by the ICP-MS analysis for the same, which was assumed as the content of Cd2+ taken up by cells. The data obtained from the replicates were taken into account to generate the data of their respective groups.
Cytotoxicity assessment
The cytotoxicity of Cd2+ was evaluated by a cell viability test using the trypan blue method to determine the loss of membrane integrity [20]. Initially, the cells were exposed to the previously established Cd2+ concentration. After the incubation, 100 μL of LS was mixed for 8 min with 100 μL of 0.4% trypan blue solution. The cell viability was determined microscopically (400 x magnification) in a Neubauer chamber, and the following two categories of cells were scored: (1) living cells that appeared uncolored or light blue and (2) dead cells with a blue color. For this assay, 300 cells were counted for each sample analyzed.
Genotoxicity Assessment
The alkaline comet assay (single cell gel electrophoresis assay) was performed to evaluate genotoxicity according to a previously described methodology [16] and in accordance with the comet assay guidelines [21]. The microscope slide analysis was performed, counting 100 cells per slide and classifying them based on damage levels from 0 (no damage) to 4 (maximum damage). The average obtained for each treatment allowed calculating the respective damage rate, considering the DNA damage scale from 0 (100 cellsx0) to 4 (100 cellsx4) as proposed earlier [22].
RESULTS
Evaluation of Intracellular Cd Content in Human Leukocytes
One of the challenges in studies on metal toxicology is to determine the quantity of the heavy metal that is capable of entering into the cytoplasm from the cell exposed to the metal. This issue is also applicable to Cd2+ and needs to be elucidated. Furthermore, it is crucial to understand if there is a constant cell entry percentage or if this entry is disproportional considering the different concentrations of exposure. Figure 1 shows an increasing intracellular Cd2+ concentration in human leukocytes exposed to CdCl2 at concentrations ranging from 1 to 25 μg/mL, which occurred in a concentration-dependent manner (p < 0.001).
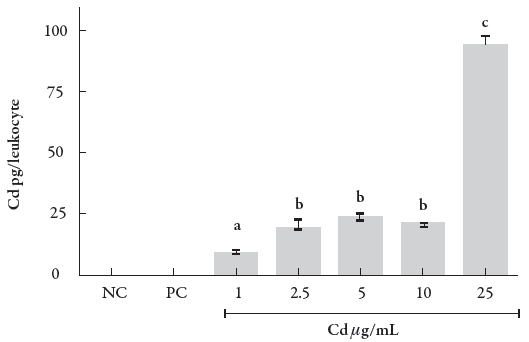
Figure 1 Intracellular Cd content in human leukocytes exposed to different concentrations of the metal. Data were analyzed by one-way ANOVA, followed by Tukey's test for multiple comparisons, and expressed as mean ± SEM, with p < 0.01. a־ b c־ d. The superscript letters indicate statistical difference between groups; NC = negative control, PC = positive control.
The percentage of Cd cellular influx equivalent to a leukocyte related to the exposure to different CdCl2 concentrations is shown in the figure 2, which allows evaluating the profile of Cd entry into a leukocyte. As shown in the figure, there is a decrease in the percentage of Cd cellular input, which occurs with a stabilization from 5 to 25 μg/mL. This phenomenon indicates a decay entry percentage but at the same time leads us to suggest that the influx kinetics is of zero-order.
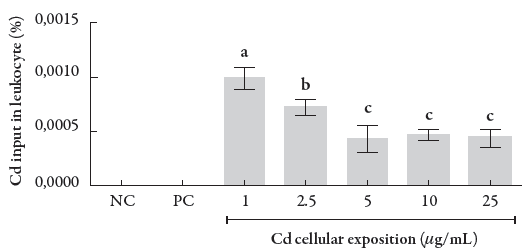
Figure 2 Percentage of cellular input of Cd in human leukocytes. Data were analyzed by one-way ANOVA, followed by Tukey's test for multiple comparisons, and expressed as mean ± SEM, with p < 0.01. a b c-the superscript letters indicate statistical difference between groups; NC = negative control, PC = positive control.
Both Cd exposure and intracellular Cd concentrations were related to the percentage of cell unviability (Fig. 3) and DNA damage index (see figure 4) in human leukocytes to elucidate the Cd concentrations capable of inducing injury in these cells. Our results demonstrated that Cd exposure at concentrations ranging from 5 to 25 μg/mL significantly decreased the leukocyte cell viability compared with the negative control (p < 0.001), in particular at two higher concentrations, 10 and 25 μg/mL.
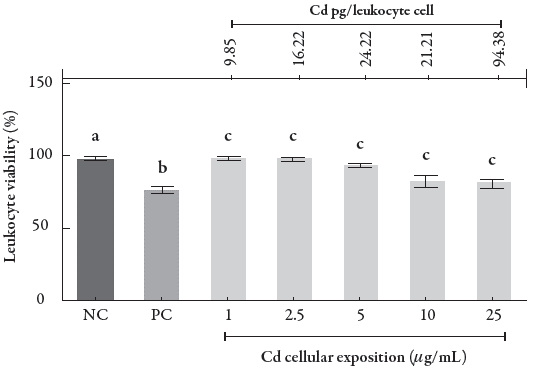
Figure 3 Percentage of cells in the cell viability assay in human leukocytes exposed to different CdCl2 concentrations. Data were analyzed by one-way ANOVA, followed by Tukey's test for multiple comparisons, and expressed as mean ± SEM, with p < 0.001; a b c. The superscript letters indicate statistical difference between groups; NC = negative control, PC = positive control.
Figure 4 shows the DNA damage index of leukocytes after CdCl2 exposure. The comet assay demonstrated that the exposure concentrations of 1 and 2.5 μg/mL, where the intracellular Cd levels were 9.85 and 16.22 pg/cell, respectively, did not cause significant DNA damage, since the damage levels were similar to the damage levels found in the negative control (0.001). On the other hand, at the exposure concentration range from 5 to 25 μg/mL, which is equivalent to the intracellular Cd content from 21.21 to 94.38 pg/cell, we were able to observe higher DNA damage levels, although they were lower than those observed for the positive control (p < 0.001).
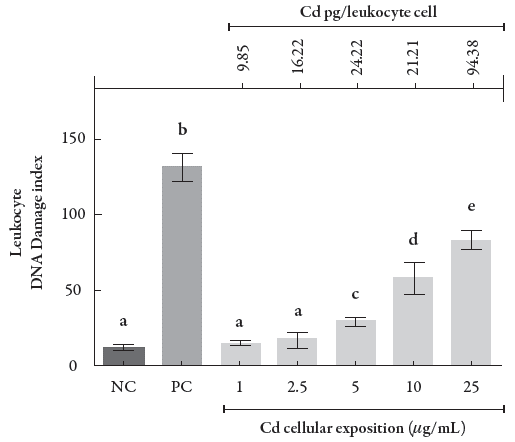
Figure 4 Rate of DNA damage in human leukocytes exposed to different CdCl2 concentrations and its respective intracellular levels following exposure. Data were analyzed by one-way ANOVA, followed by Tukey's test for multiple comparisons, and expressed as mean ± SEM, with p < 0.001. a־ b־ c־ d - the superscript letters indicate statistical difference between groups; NC = negative control, PC = positive control.
DISCUSSION
Cd2+ is a nonessential heavy metal and one of the most relevant toxic pollutants associated with environmental and public health, which is due to its high water solubility and bioaccumulation capacity in living organisms [23,24]. Furthermore, Cd2+ is potentially carcinogenic and teratogenic, causing several abnormalities [24,25]. Cd2+ also contributes to the genotoxicity of other agents that may cause damage to the DNA structure [25,26].
The cellular unviability and comet assays are techniques that are capable of detecting damages to cells and DNA, respectively. The literature reports different data about Cd2+ pertaining to cellular and DNA damages at different concentrations [27-29]. However, in this study, we demonstrated, using an unprecedented strategy, that the cellular and DNA damages begin to appear at concentration from 5 μg/mL of CdCl2 considering a short exposure of 1 h.
There are several studies demonstrating Cd2+ toxicodynamic mechanisms. These studies address different molecular targets such as the increase in oxidative stress, evidenced by the increase in catalase and superoxide dismutase activities, besides the increase in malondialdehyde levels and the depletion of GSH concentrations [30-32]. In addition, Cd2+ exposure increases the cytosolic calcium levels under homeostatic conditions, i.e., at 0.1 μΜ [34]. This event triggers cellular destruction through the activation of phospholipases, endonucleases, proteases, and Ca+2/Mg+2 ATPases dependent on the energy associated to the membrane [33-35].
Consistent with this, some studies corroborate with the apoptotic mechanisms deflagrated after Cd2+ exposure of crab hepatopancreas (30-57 mg/mL) and cortical neurons of rats (20-25 μΜ for 24-48 h), where it was possible to observe an increase in the activity of caspases 9 and 3, but not caspase 8, associated with a decrease in cytochrome c activity and an increase in the expression of poly-ADP-ribose-polymerase (PARP) [28, 29]. These data corroborate with the idea that cellular and DNA damages may be induced by free radicals after Cd2+ exposure, since cytochrome c is activated and released to activate caspase 9, especially through free radical generation. Notwithstanding, PARP is closely associated with cellular and DNA repair mechanisms, which is used as a biomarker for such.
In addition, human lungs cell strain (A549 and H441) were exposed to 20 and 40 μΜ of CdCl2 that resulted in an increase in the activities of metallothionein (MT), HSP70, and MDR1. These data corroborate with the above-cited whether considered the mechanisms of auto defense triggered by cells under injuries against their aggressors, as the Cd2+. In this case, the cell attempts to neutralize Cd2+ by binding with MT; decrease the effects of stress oxidative through the HSP70, as well as to participate of protein folder; or increase the transport of metal efflux through the MDR1. However, these cytoprotective events are not effective when the metal concentrations are higher than their capacity to neutralize them [27].
The Cd2+ accesses the inside of the cells competing with the input pathways via ion channels, solutes carriers or ATP-requiring pumps. Once in the cell, Cd2+ can exert its toxic effects by binding to the thiol compounds scattered throughout the cell (cyto-sol and nucleus), as observed in the disruption respiratory chain function by binding Cd2+ to mitochondrial thiol proteins. In addition, Cd2+ may bind to the amino acid-cystine transporters (SLC79/SLC3A1 and SLC7A9/SLC7A13) to access the cytosol. Besides, it is accepted that Cd2+ has direct interaction with cellular DNA, is capable of inhibiting the nuclease of incision during DNA repair, as well as be able to exert an interference in the chromatin condensation [36].
Based on the abovementioned assumptions, the damage induced by Cd2+ in different types of cells is dependent on concentration and time of exposure. However, till date, no study has yet demonstrated the range of intracellular Cd2+ concentrations that could induce cellular damage. Although we are not aware of the fact whether intracellular Cd2+ concentration resulting from the exposure of 5 μg/mL has the same profile of cellular input, we can confirm that in human leukocytes, under the evaluated conditions, the Cd2+ content of 33 pg/leukocyte is responsible to induce cytotoxicity and genotoxicity. Furthermore, the intracellular Cd2+ percentages resulting from the exposure concentrations in leukocytes suggest a profile of zero-order kinetics, which may be associated with the saturation of a specific protein domain for the binding of metals in cell membranes.
CONCLUSION
Our results provide new insights regarding the concentration, exposure, and Cd2+ (CdCl2) that are capable of inducing cell cytotoxicity and genotoxicity in human leukocytes. Furthermore, our study provides a better understanding about in vitro Cd2+ as a source of inducing cytotoxicity and genotoxicity at the exposure concentrations ranging from 5 to 25 μg/mL, with the intracellular levels ranging from 21.21 to 94.38 pg/cell, respectively. Using an unprecedented strategy, the intracellular Cd2+ level was correlated with the different exposure concentrations, and based on this, we could deduce that the Cd inflow occurs under zero-order kinetics.















