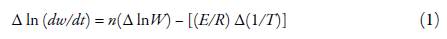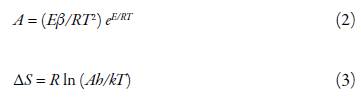INTRODUCTION
Thermal analysis has been used to determine the physical and chemical properties of various types of materials [1-6]. Scientific and technological achievements together with demands based on industrial requirement have permitted the development of various types of materials that can withstand at much higher temperatures and more corrosive environments. It is widely used in various fields to study composition analysis, product reliability, stability, chemical reaction and dynamic properties [7-9]. The analysis is popular in various industries namely pharmaceutical [10, 11], forensic [12, 13], food [14, 15], ceramics [16, 17], polymer [18, 19], composites [20, 21] and semiconductors [22] industries. Further, by thermal analysis, kinetic parameters of thermally simulated reactions can be evaluated which provides a deeper insight in to the mechanism of high energetic compounds [23-26].
Literature survey shows that thermal analysis has been reported for a variety of materials such as coals, rocks, minerals, nano-materials, rubber, styrene butadiene blends, polyimide resins, superconducting materials, hydrogen storage materials, rocks from moon, hydrocarbon sludge etc. [27-30]. Thermal analysis can be done by various techniques such as differential scanning calorimetry, differential thermal analysis, thermogravimetric analysis, evolved gas detection, evolved gas analysis etc.
Among many heterocyclic compounds, pyrimidine compounds are one of the most prominent structures which attract many chemists and pharmacists due to their therapeutic values. Further, many of these compounds are known to exist in deoxyribonucleic acid and ribonucleic acid which is one of the most essential constituents of all cells and thus of all living matter. As many of these compounds exist in various drugs [31-33], it would be useful to study their thermal stability and other thermal parameters which are prime factors for the application and shelf life of a drug.
Thus, in the present work, thermal properties of some newly synthesized pyrimidine derivatives such as dihydropyrimidinones, dihydropyrimidinthiones, tetrahydropyrimidines and 2, 4-disubstituted pyrimidines have been studied by thermogravimetric (TG) and differential scanning calorimetric (DSC) techniques. From TG thermograms, the thermal stability and various kinetic parameters such as order of the degradation, energy of activation, frequency factor and entropy change have been evaluated. The purity of synthesized compounds has been checked by DSC which also gives the melting points and heat of fusion of the studied compounds.
EXPERIMENTAL
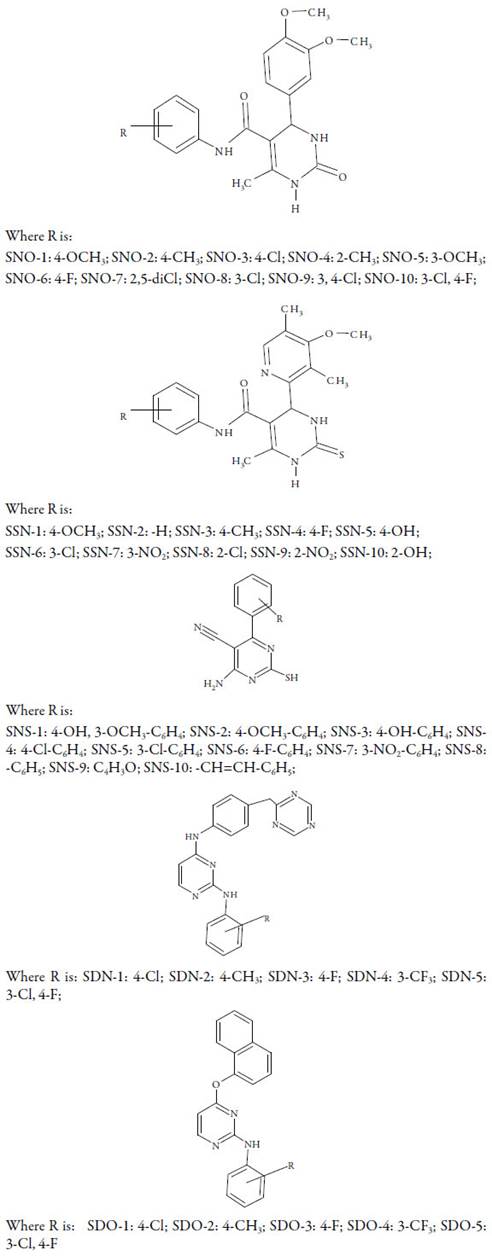
A wide variety of pyrimidine compounds have been synthesized. The general structures and substitutions in different compounds are given in figure 1.
RESULTS AND DISCUSSION
Thermal stability
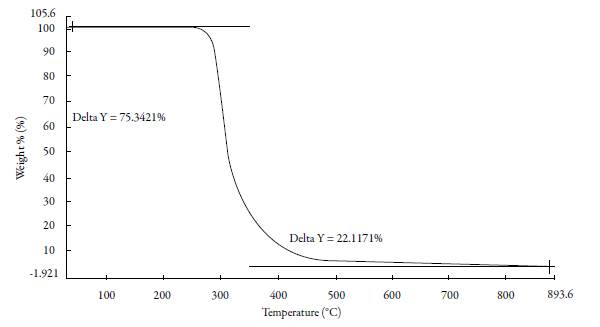
Figure 2 shows the TGA thermogram of a single pyrimidine compound SSN-1. Various thermal properties such as decomposition temperature range, percentage weight loss and residual weight are reported in table 1 for all the compounds.
Table 1 TGA data for synthesized compounds.
| Comp. Code | Amount (mg) | Decomposition Range (°C) | % Weights loss | Residual wt. loss (mg) |
| SNO Series | ||||
| SNO-1 | 0.933 | 150-700 | 97.96 | 0.913 |
| SNO -2 | 2.714 | 170-550 | 90.69 | 2.461 |
| SNO -3 | 3.247 | 165-480 | 59.86 | 1.943 |
| SNO -4 | 2.527 | 175-450 | 58.81 | 1.481 |
| SNO -5 | 2.616 | 145-510 | 58.85 | 1.539 |
| SNO -6 | 9.477 | 210-610 | 57.33 | 5.433 |
| SNO -7 | 7.571 | 150-380 | 84.05 | 6.363 |
| SNO -8 | 4.175 | 210-485 | 72.50 | 3.026 |
| SNO -9 | 5.834 | 100-500 | 75.37 | 4.396 |
| SNO -10 | 8.559 | 100-490 | 52.01 | 4.472 |
| SSN Series | ||||
| SSN-1 | 2.230 | 188-621 | 99.74 | 2.222 |
| SSN -2 | 5.827 | 280-792 | 98.75 | 5.754 |
| SSN -3 | 1.490 | 255-824 | 98.80 | 1.472 |
| SSN -4 | 2.727 | 230-665 | 99.21 | 2.705 |
| SSN -5 | 2.916 | 167-820 | 81.76 | 2.384 |
| SSN -6 | 4.922 | 125-548 | 86.24 | 4.244 |
| SSN -7 | 9.423 | 200-600 | 61.31 | 5.777 |
| SSN -8 | 7.493 | 178-400 | 79.74 | 5.974 |
| SSN -9 | 4.406 | 146-400 | 65.44 | 2.883 |
| SSN -10 | 5.800 | 98-500 | 64.94 | 3.766 |
| SNS Series | ||||
| SNS-1 | 5.285 | 168-311 | 95.55 | 5.050 |
| SNS-2 | 4.614 | 133-281 | 96.36 | 4.446 |
| SNS-3 | 0.912 | 159-281 | 94.76 | 0.8642 |
| SNS-4 | 6.505 | 100-200 | 97.24 | 6.326 |
| SNS-5 | 4.009 | 83-161 | 97.50 | 3.909 |
| SNS-6 | 3.401 | 125-234 | 99.34 | 3.378 |
| SNS-7 | 2.103 | 169-287 | 64.55 | 1.3575 |
| SNS-8 | 2.734 | 123-233 | 98.46 | 2.692 |
| SNS-9 | 2.105 | 97-205 | 99.07 | 2.086 |
| SNS-10 | 2.656 | 78-418 | 64.48 | 1.7125 |
| SDN Series | ||||
| SDN-1 | 3.361 | 80-800 | 88.16 | 2.963 |
| SDN-2 | 4.716 | 105-735 | 98.79 | 4.665 |
| SDN-3 | 2.360 | 110-630 | 96.59 | 2.279 |
| SDN-4 | 2.856 | 140-680 | 97.96 | 2.798 |
| SDO Series | ||||
| SDO-1 | 2.791 | 90-635 | 98.53 | 2.750 |
| SDO-2 | 1.513 | 120-600 | 96.69 | 1.463 |
| SDO-3 | 1.765 | 90-525 | 88.44 | 1.561 |
| SDO-4 | 2.961 | 95-530 | 96.00 | 2.842 |
| SDO-5 | 2.907 | 105-610 | 95.54 | 2.777 |
SNO series.
For some compounds, degradation is single step process whereas for others, it is multi step process. For SNO-1 and SNO-3, degradation is multi step process whereas for other compounds, it is single step. Table 1 show that SNO-9 and SNO-10 is most unstable and SNO-6 and SNO-8 are stable. As evident from figure 1, substitutions are different in different compounds although central moiety is same. SNO-10 contains 3-chloro and 4-fluoro groups whereas SNO-9 has two chloro groups at 3 and 4 positions. SNO-6- contains 4-fluoro group whereas SNO-8 contains only 3-chloro group.
Thus, the number and position of group also affect the stability of compound. Other compounds containing different other substitutions have intermediate thermal stability.
SSN series.
For most of these compounds, degradation is single step process. However, for compounds SSN-1 and SSN-6, multi-step degradation takes place. It is clear from table 1 that SSN-10 is most unstable and SSN-2 is most stable. SSN-10 contains 2-OH group whereas SSN-2 is without substitution. Thus, the absence of any functional group to aryl ring increases the stability. The decomposition continues up to approximately 800 and up for SSN-2, SSN-3 and SSN-4. SSN-2 is without any functional group. SSN-3 and SSN-4 contain 4-methyl and 4-fluoro groups respectively which increase the decomposition temperature. Comparison of SSN-5 and SSN-10 shows that SSN-5 is more stable than SSN-10. Both these compounds contain hydroxyl group. In SSN-5, it is at 4th position whereas in SSN-10, it is at 2nd position. Similarly, SSN-6 and SSN-8 contain chloro group at 3rd and 2nd positions respectively. However, the decomposition temperature range for these compounds is different. In SSN-7 and SSN-9 compounds, nitro group is present at 4th and 2nd positions respectively. In this case, SSN-9 is unstable than SSN-7. Again, decomposition range is higher for SSN-7 containing 3-nitro group. This suggests that position of functional groups also affect the stability and the presence of group at 2nd position decreases the stability.
SNS series.
For all the compounds, degradation is single step process and degradation temperature is less than 200 °C. Out of ten compounds, SNS-10 is most unstable which is followed by SNS-5. SNS-7 is the most stable compound which is followed by SNS-1. SNS-10 contains cinnamaldehyde as substitution to aromatic ring. Thus, cinnamaldehyde decreases the thermal stability. The presence of nitro group at 3rd position increases the stability as in SNS-7. The variation in thermal decomposition may also be due to some intermolecular interactions (structural as well as electronic).
SDN series.
For all the compounds, degradation is multi step process. It is clear from table 1 that for SDN series compounds, SDN-1 is most unstable and SDN-4 is most stable compound. SDN-4 contains 3-CF3 whereas SDN-1 contains 4-chloro substitution. Thus, substitution affects the thermal stability of a compound. Overall, the dominating effect of different group is: positive resonating (+R) > positive hyper conjugation effect (+H) > negative inductive In the present study, order of effect of different substitution is 4-Cl > 4-CH3 > 4-F> 3-CF3. The more stability of SDN-4 may be due to negative inductive effect of 3-CF3. Whereas due to positive resonating effect of 4- CH3 group, SDN-1 is most unstable.
SDO series.
Table 1 shows that SDO-2 is most stable while SDO-1 and SDO-3 are unstable. Thus, the presence of 4-CH3 group increases the stability. SDO-1 and SDO-3 contain 4-chloro and 4-fluoro group respectively which causes decrease in stability. However, when a compound contains both chloro and fluoro groups as in SDO-5, stability increased.
Comparison of SDN and SDO series suggests that structure affects decomposition. The central moieties are different in both series but side chains are same. In SDN series, 3-CF3 increased the stability whereas in SDO series, it decreased the stability.
Overall comparison of synthesized compounds shows that a substitution in a particular moiety increases thermal stability whereas the same substitution in other moiety causes decrease of stability. This suggests that the variation in thermal decomposition may also be due to some intermolecular interactions.
Kinetic parameters
Further, from these thermograms, various kinetic parameters, such as order of the degradation, energy of activation, frequency factor and entropy change have also been evaluated using Anderson-Freeman equation [34]:
where dw/dt is the rate of decomposition, Wis the active mass, R is gas constant and T is temperature. n is order of degradation and E is energy of activation.
The frequency factor (A) and the entropy change (∆S) were determined by following equations [35]:
where ( is heating rate (10°C per minute), h is Planck's constant and k is Boltzmann constant.
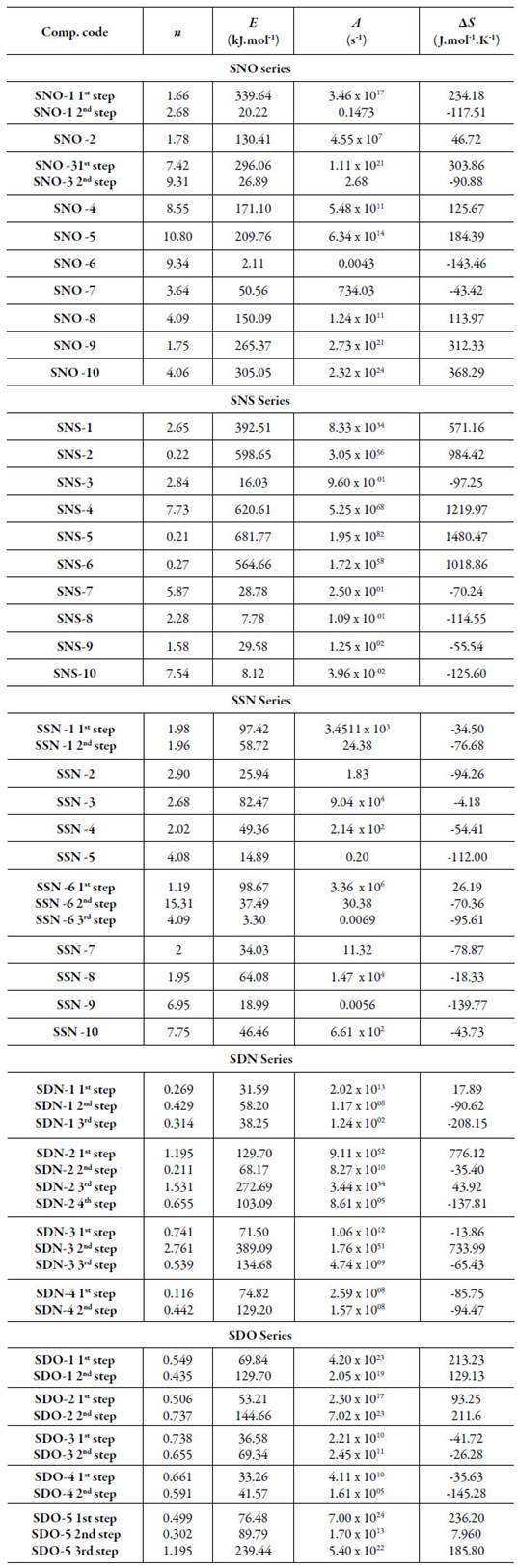
All the evaluated kinetic parameters are listed in table 2 for all the compounds.
It is evident from table 2 that order of reactions is quite different in different compounds and in different steps. There is wide range of values of energy of activation (E), frequency factor (A) and entropy change (∆S) for studied compounds.
SNO series.
Table 2 shows that the order of reactions is quite different in different steps for different dihydropyrimidinones. The order of reaction varies from 1.75 to 10.80 for single step degradation. For multistep degradation, it varies from 2.68 to 9.31. For single step degradation compounds, energy of activation is maximum for SNO-10 and minimum for SNO-6. The frequency factor also varies in the same order. For multi-step degradation compounds, in the first step, energy ofactivation is maximum for SNO-1 whereas in second step, it is maximum for SNO-3. The frequency factor is maximum for SNO-3 in first step and minimum for SNO-1 in second step.
Further, change in entropy (∆S) for all the compounds is both positive and negative. The negative ∆S values indicate more ordered or more rigid structure whereas positive AS values indicate that the transition state is in less ordered state [36]. The highest value of entropy is for SNO-6 which has lowest energy of activation and frequency factor. The same but opposite order is for SNO-10 for single step degradation.
SSN series.
For single step decomposition, order of reaction varies from 1.95 to 7.75. For SSN-1, the order of reaction is almost same for both steps, difference is only of 0.02. However, much change is observed for different steps in SSN-6.
For single step degradation, energy of activation (E) is maximum for SSN-3 and minimum for SSN-5. The values of frequency factor (A) are quite different in first step. The frequency factor is maximum for SSN-3 and minimum for SSN-9. For multi-step degradation, in both SSN-1 and SSN-6, energy of activation and frequency factor is higher for the first step.
The entropy change is negative for all compounds except SSN-6 for the first step. The negative entropy indicates that the activation compound has a more ordered or more rigid structure than the reactants and reaction is slower than the normal whereas positive entropy indicates that the transition state is in less ordered state.
SNS series.
The order of reaction (n) varies from 0.21 to 7.54. The value of n is minimum for SNS-2 and maximum for SNS-4. The energy of activation (E) is highest for SNS-5 containing chloro group at 3rd position and minimum for SNS-8 which is without any substitution. The frequency factor (A) is also highest for SNS-5 but minimum for SNS-10. The entropy change is found to be both positive and negative.
SDN series.
The order of reaction is different in different steps for different compounds. For some compounds, it is less than one for most of the steps. The maximum order of reaction is found to be 2.761. However, for all the compounds, it is less than one for all the steps except 3rd step of SDN -5. The energy of activation (E) is highest for SDN-3 in second step while it is minimum in first step of SDN-1.
SDO series.
In SDO series, the energy of activation is highest in third step of SDO-5 while lowest in first step of SDO-4. The frequency factor is highest for first step of SDO-2 and lowest for third step of SDO-1 compound. In SDO series, it is found to be maximum in first step of SDO-5 and minimum in second step of SDO-4 compound.
The entropy change is quite different for different compounds and the values are both positive and negative for different compounds. The positive values indicate that the transition state is less ordered than the original compound whereas negative entropy corresponds to an increase in the order of transition state than that of individual molecules.
From DSC, melting points of all the compounds are determined and are given in Table 3 along with melting points determined by open capillary method. There is good agreement between the values evaluated from DSC and those determined by open capillary method.
Table 3 The melting temperatures (°C) of synthesized compounds by DSC and open capillary methods.
| Compound code | DSC (°C) | Open capillary (°C) |
| SNO series | ||
| SNO-1 | 168.81 | 168 |
| SNO -2 | 153.72 | 152 |
| SNO -3 | 181.01 | 179 |
| SNO -4 | 181.55 | 182 |
| SNO -5 | 162.84 | 164 |
| SNO -6 | 158.84 | 160 |
| SNO -7 | 170.27 | 171 |
| SNO -8 | 199.11 | 198 |
| SNO -9 | 168.83 | 169 |
| SNO -10 | 144.15 | 145 |
| SNS series | ||
| SNS -1 | 138.89 | 140 |
| SNS-2 | 119.32 | 118 |
| SNS-3 | 190.57 | 190 |
| SNS-4 | 167.13 | 168 |
| SNS-5 | 156.85 | 157 |
| SNS-6 | 130.87 | 130 |
| SNS-7 | 98.68 | 101 |
| SNS-8 | 89.82 | 90 |
| SNS-9 | 79.86 | 81 |
| SNS-10 | 162.17 | 164 |
| SSN series | ||
| SSN -1 | 179.20 | 180 |
| SSN -2 | 193.62 | 194 |
| SSN -3 | 210.31 | 211 |
| SSN -4 | 253.14 | 254 |
| SSN -5 | 192.87 | 194 |
| SSN -6 | 167.74 | 167 |
| SSN -7 | 233.34 | 235 |
| SSN -8 | 246.60 | 247 |
| SSN -9 | 237.12 | 238 |
| SSN -10 | 206.81 | 208 |
| SDN series | ||
| SDN-1 | 122.74 | 123 |
| SDN-2 | 153.91 | 153 |
| SDN-3 | 191.76 | 193 |
| SDN-4 | 201.55 | 202 |
| SDN-5 | 212.95 | 214 |
| SDO series | ||
| SDO-1 | 158.05 | 160 |
| SDO-2 | 294.60 | 294 |
| SDO-3 | 292.18 | 292 |
| SDO-4 | 249.24 | 250 |
| SDO-5 | 147.27 | 148 |
Comparison of thermal data of different series shows that if in one series a particular substitution increases the thermal stability, in the other series, it decreases.
CONCLUSIONS
It is concluded that thermal stability depends on structure of compound as well as substitutions. The position of substitution in aromatic ring skeleton also affects thermal stability and kinetic parameters. The kinetic parameters differ greatly for different compounds. No correlation could be established between kinetic parameters, melting temperature, thermal stability and substitution groups.