INTRODUCTION
In general terms, raw propolis is composed of 50% of resin and vegetal balsam, that contains the most of biologically active substances; 30% to 35% of waxes; 5% to 10% of aromatic oils; approximately 5% of pollen and at lower proportion, organic waste. Additionally, in propolis there are sodium, calcium, magnesium, zinc, potassium, silicon, iron, sulfur and aluminum [1], and vitamins such as thiamin, riboflavin, pyridoxine, ascorbic acid, and α-tocopherol [2-4].
As one of the bee products with higher biological and pharmacological activity, due to the diversity and a large number of its compounds [5, 6], the medicinal and personal-care uses of propolis have been reported since ancestral times. Approximately, more than three hundred different chemical compounds are identified in this material including flavonoids, phenolic acids, acetophenone derivatives, lignans, terpenes, volatile components (such as monoterpenes and sesquiterpenes), aromatic compounds, sugars, hydrocarbons, and monoesters [5, 7]. Thus, up to now, there is evidence of propolis activity as antimicrobial [8-10], antifungal [11, 12] and antiviral [13, 14].
However, the composition of propolis depends on the geographical location of the beehive because of variations of the exudates and the secretions of the surrounding plants [2, 15]. Likewise, seasonality, illumination, altitude, race of bees, method of propolis harvesting and parallel activity in the zone of the hive location influence the propolis composition [15]. On this basis, propolis could be harnessed in many ways at an industrial level depending on its quality. For this reason, this research work deeps on the quality of two samples of Colombian propolis as a starting point for identifying their potential applications in pharmaceutics and cosmetics. It is the interest to increase the awareness of propolis as a natural resource and to provide readers with criteria for taking advantage of its composition with a view to developing innovative products from this raw material.
MATERIALS AND METHODS
Materials
The first raw propolis sample (PrS) used in this study was collected from hives of stinging bees,Apismellifera, in Confines, Santander, Colombia (6°21'21.0"N 73°14'27.0"W, altitude: 1498 masl). The second raw propolis sample (PrJB) was kindly donated by the Joaquín Antonio Uribe Botanical Garden of Medellín, Antioquia, Colombia (6°16'15.0"N 75°33'51.0"W, altitude: 1486 masl), collected from hives of stingless native bees, Tetragonisca angustula. Both propolis samples were stored at -20 °C in plastic containers.
Others materials used in this research work include ethyl acetate (EtAc; Tedia"), n-hexane (J.T.Baker), methanol (MeOH; J.T.Baker), pharmaceutical grade ethanol 96% (Chemi), chloroform (Aldrich), sulfuric acid (Mallinckrodt), aluminium chloride 6-hydrate (Panreac), quercetin (Sigma-Aldrich, purity ≥ 95%, Lot SLBD8415V), sodium nitrite (Merck), 2-aminoethyl diphenylborinate (Sigma-Aldrich), PEG 4000 (Sigma-Aldrich), sodium sulfate anhydrous (Carlo Erba), vanillin (Merck), anisalde-hyde (Carlo Erba). For analyzing the samples of Thin Layer Chromatography (TLC), TLC Silica Gel 60 F254 plates (Merck Millipore) were used as stationary phase and as a mechanism of detection Godin's reagent [aspersion of1% ethanolic vanillin (Merck), 10% ethanolic sulfuric acid (Mallinckrodt)], anisaldehyde-sulfuric acid reactive [0.5 mL of anisaldehyde (Carlos Erba), 10 mL of glacial acetic acid (Mallinckrodt), 85 mL of MeOH (J.T. Baker), 5 mL of 96% sulfuric acid (Mallinckrodt), and natural products - polyethylene glycol reactive (NP-PEG) [mixture of 1% methanolic diphe-nylboriloxietilamine (NP, Sigma-Aldrich) and 5% ethanolic polyethylene glycol 4000 (PEG 4000, Sigma Aldrich) at a ratio 5:4]. Distilled water was used in all experiments (Boeco distiller WS8000).
Methods
As shown in figure 1, different parameters were included for investigating the quality of propolis. The used methodologies are based on standard methods stated by the Association of Official Agricultural Chemists (AOAC) and the United States Pharmacopoeia (USP), or by procedures reported in different published research works.

Figure 1 A synthesis of the tests used for characterizing raw propolis. Procedures carried out on raw propolis /procedures requiring propolis extraction.
Organoleptic characterization: Propolis was evaluated in its odor, color, flavor, consistency, and aspect at room temperature.
Melting range: A small quantity of raw propolis was located at the capillary tube of a melting point apparatus (Bùchi SMP-20) having silicone as a heating medium. The starting and ending temperature of the melting process were recorded [16] and the results are reported as the mean of five independent measurements with a 95% confidence interval.
Waxes or n-hexane extractable material: Raw propolis (2 g) was extracted by the Goldfish method with 90 mL of n-hexane for 6 h [17]. The content of waxes corresponds to the remaining material in the flat-bottom flask, once the extraction process was finished and all the organic solvent was removed by evaporation (oven Heraeus Instruments). The n-hexane extractable material is reported as a percentage of the raw propolis sample from the mean of two independent measurements with a 95% confidence interval.
Soluble residue in methanol (dry residue): As reported by Funari and Ferro [18], 10 g of propolis were treated for 8 h with approximately 150 mL of methanol by using the Soxhlet method. The extract was transferred in a graduated cylinder taking care of rinse the used flat-bottom flask with methanol. The final volume of the extract was measured, and it was stored in an amber recipient in a refrigerator (2°C) for 24 h and then, in a freezer (-20°C) for 0.5 h. Afterward, it was filtered in a Büchner funnel using Boeco qualitative filter paper No. 3. A 5-mL aliquot of the wax-free methanolic extract was transferred to a crucible (previously dried at 105 °C for 2 h, cooled in a desiccator and weighted). The set was dried in an oven at 105 °C for 2 h, cooled in a desiccator and weighted. The procedure was repeated up to constant weight. The soluble residue in methanol was reported as a percentage of the raw propolis from the mean of six independent measurements, with a 95% confidence interval.
Ethanol-soluble compounds or percentage of ethanolic extract: The determination of resins was carried out as reported by Bedascarrasbure et al. [17]. Thus, the remaining raw propolis sample once the test of n-hexane extractable material was finished, was extracted with ethanol using the Goldfish extraction apparatus. For this purpose, about 90 mL of ethanol were added to the 250-mL flat-bottom flask and the boiling of solvent was allowed; these work conditions were maintained for 6 h, with a flow rate of 120 to 150 drops of condensate per min. The extraction process finished when the ethanol was colorless. As additional checking, a drop of ethanol was added to 2 mL of a 10% ferric chloride solution; if the color changed from yellowish to greenish, the presence of phenolic compounds was confirmed, and the extraction must continue.
When the extraction process finished, the ethanolic solution was quantitatively transferred to a 100-mL volumetric flask and the volume was completed with ethanol. Then, an aliquot of 50 mL was distilled in a bath at 80 °C, the residue was dried at the same temperature (oven Heraeus Instruments) and cooled in a desiccator. The drying and cooling processes were repeated up to constant weight. The obtained residual corresponds to the ethanol-soluble compounds that were reported as a percentage of the raw propolis sample. The results are reported as the mean of two independent measurements with a 95% confidence interval. The remaining ethanolic extracts of the two replicates were mixed and stored in a 100-mL amber recipient for additional determinations.
Mechanic impurities or foreign bodies: For determining the mechanic impurities in propolis the procedure reported by Bedascarrasbure et al. [17] was followed. To this end, the remaining raw propolis sample once the tests of n-hexane extractable material and ethanol-soluble compounds were finished, was dried in an oven (Heraeus Instruments) at 80 °C for 1 h, cooled in a desiccator and led to constant weight. The mechanic impurities were reported as a percentage of the raw propolis sample from the mean of two independent measurements with a 95% confidence interval.
Loss on drying: Propolis sample was weighted (3.0 g) into a Petri dish that was previously stored in an oven at 135°C for 1 h, cooled in a desiccator and weighted. The set was drying at 135°C ± 2°C for 4 h (oven Heraeus Instruments) and cooled at room temperature in a desiccator. The procedure was repeated until a constant weight was reached [19]. The loss on drying was expressed as the percentage of weight loss of the sample and the results are reported as the mean of three independent measurements with a 95% confidence interval.
Ash: To determine the content of ash in propolis, the AOAC protocol was followed with some modifications [20]. In this way, 3.0 g of propolis were weighted into a porcelain crucible (that was previously dried at 550°C for 1 h, cooled at desiccator and weighted). A slow pre-calcination of the sample was done on a heating plate (IKA" C-MAG HS 7) and then, it was calcinated at 550°C for 12 h (muffle Hotpack) to obtain white ashes. The set was removed, cooled in a desiccator at room temperature and weighted. Ash content is expressed as a percentage and the results are reported as the mean of three independent measurements with a 95% confidence interval.
Lead: The Method II of the USP 38 [21] was used for the detection of lead in a limit assay (2 ppm), utilizing a propolis sample of10 g.
Arsenic: Digestion of propolis (0.5 g) was carried out by reflux with concentrated HNO3 (5 mL) until digestate is light in color and it does not change by visual inspection. Then, digestate was diluted to 10 mL. The quantitative analysis by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES; ICP spectrometer Icap 7400, Thermo Scientific; optic system type Echelle polychromator; detector in solid state CID -Charge Injection Device-; software: QTEGRA) was carried out following the '"StandardMethods', 3120 A, B reported by Rice, Baird, Eaton and Clesceri [22]. Argon gas ionized by radiofrequency was used as a source of plasm working at a temperature near to 9000 K. Digested propolis was analyzed to 189.042 nm with a calibration blank and a method blank. The quantification was made using arsenic internal standard. Instrumental detection limit, precision, optimum background correction position, linear dynamic range and correction for interferences were established. The routine procedures for the test quality control were executed (e.g. checking the memory effect and verification with the standards of continuous calibration).
Oxidation index: A ground propolis sample (0.2 g) was placed in a 125-mL erlenmeyer and 5 mL of 96% ethanol were added, and after 1 h, 100 mL of distilled water were added. The obtained dispersion was filtered (Boeco qualitative filter paper No. 3). In a test tube 2 mL of filtered were added with 1 mL of 20% sulfuric acid and all was stirred in a vortex for 1 min; then, a drop of 0.1 N KMnO4 was added and the time of discoloration was measured [17]. The result is reported as the mean of six independent measurements with a 95% confidence interval.
TLCcharacterization: Propolis extracts were tracked by TLC using chloroform: EtAc (70:30) as mobile phase and visualized by NP-PEG and Godin's reagent.
Identification of phenolic compounds: In this test, the ethanolic extract obtained in the ethanol-soluble compounds test was also used. Thus, a dilution of that extract was prepared with ethanol (e.g., 1 to 1000) so that absorbances between 0.5 to 0.8 units were read at 290 nm. Then, scanning was done between 240 nm and 420 nm. It was considered that phenolic compounds were present in absorption maxima between 270 nm and 315 nm [17].
Content of phenolic compounds: The total phenolic compounds were determined following the Folin-Ciocalteau method [23-25]. For the sample processing, 0.5 g of ground propolis and 15 mL ethanol were placed inside a 100-mL beaker. The mixture was protected from light, stirred with a magnetic bar at 500 rpm for 30 min, and stored during 24 h at room temperature. The obtained extract was filtered and transferred to a 50-mL volumetric flask; this solution was kept in freezing at -20 °C until its analysis. To measure phenolic compounds, 500 µL of ethanolic propolis extract were added with 500 µL of Folin-Ciocalteu reagent; after 2 min, 2 mL of 10% sodium carbonate solution were also added, and the volume was completed to 50 mL with distilled water. The sample was stored during 2 h protected from light and the spectrophotometric measurement was carried out at 765 nm with distilled water as blank (Genesys* 10S UV-Vis Spectrophotometer; calibration line with gallic acid, range: 0.02 - 1.0 mg/mL; r2: 0.999). The total content of phenolic compounds is expressed as the mean of the gallic acid equivalent milligrams from nine independent measurements with a 95% confidence interval.
Content of flavonoids: The flavonoids in propolis were determined according to Bedas-carrasbure et al. [17] and Woisky and Salatino [26] with some modifications. In brief, 1 mL of ethanolic propolis extract (10 mg/mL) was mixed with 0.5 mL of 5% AlCl3 methanolic solution and completed to 10 mL with methanol. A blank solution was similarly prepared using 96% ethanol instead of the sample. The solutions were stored in darkness for 30 min and their absorbance was spectrophotometric determined at 425 nm (calibration line with quercetin dihydrate, range: 1.2 - 15.1 microgramos/mL; r2: 0.999). The content of flavonoids is expressed as the percentage of quercetin from two independent measurements with a 95% confidence interval.
Antioxidant activity: To this end, the Trolox Equivalent Antioxidant Capacity (TEAC) test reported by Ahn et al. [27] was followed with some modifications. Thus, the absorbance of the ABTS+radical working solution (1 mL) was read at 734 nm, using ethanol as blank. Immediately, 10 microlitros of the ethanolic propolis extract, obtained in the test for phenolic compounds, were added to the working solution, the system was stirred, and the absorbance of the mixture was measured after 6 min. The difference in absorbances between the initial time (zero minute) and the final time (six minute) was expressed in mM of Trolox/g of propolis by using a calibration line of Trolox, i.e., 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (range: 0.2 mM and 2.0 mM, r2: 0.993) and from the mean of nine independent measurements with a 95% confidence interval.
RESULTS AND DISCUSSION
As mentioned, propolis is a raw material of natural source highly valued in pharmaceutics and cosmetics because of its content of active molecules. However, to make statements about the harnessing of propolis in these fields is not easy considering the different factors determining its quality and purity and the approximately 17500 species of bees reported worldwide [28], of which approximately one thousand collect propolis [29]. Only in Colombia, nearly 466 species of bees have been listed [30] where variations in quality are demonstrated [31].
Therefore, in this work, an approach based on the typical quality controls of propolis is used to propose its potential applications in the design of pharmaceutical and cosmetic products. Although there is a large number of test to be carried out to this end [32], in this work the physicochemical properties of propolis (organoleptic characterization, melting range and loss on drying), its purity (ash, mechanic impurities, n-hexane extractable material, lead, and arsenic), and the general characterization, identification or quantification of active compounds (ethanol-soluble compounds, oxidation index, TLC characterization, identification and quantification of phenolic compounds, content of flavonoids and antioxidant activity) were chosen.
To interpret the results, the Brazilian legislation [33, 34] and the Argentine legislation [35] were used as reference considering the geographical proximity with Colombia (see table 1). Also, USP specifications were taken into account due to our pharmaceutical and cosmetic approach.
Table 1 Quality control sheet of two samples of Colombian propolis.
*1[33,34]. 2[35].3[21]. *NMT: no more than **NLT: no lower than.
Physicochemical properties of propolis
In general, propolis is described as a malleable to rigid material of homogeneous or heterogeneous aspect, with characteristic flavor (from mild balsamic to strong and spicy) and characteristic odor (resinous or balsamic). The propolis color may well be varied according to the origin, but yellow, brown, greenish, reddish are the most common ones.
As shown in table 1, the investigated samples of Colombian propolis are similar in appearance; they are of heterogeneous aspect, rigid consistency, with melting range around 60 °C, dark brown color and resinous odor. Differences are detected in flavor, which varies from slightly spiced (PrS) to balsamic (PrJB). On the other hand, both samples comply with the reference standards about the loss on drying, i.e., the content of volatile matter including water.
Purity quality control of propolis
The evaluation of purity for a raw material intended to be used in the preparation of drugs and cosmetics include different tests that are chosen considering its origin. For propolis, the residue of ignition evidences its mineral content, which is a criterion to identify possible adulterations, especially for propolis samples supplied as a dry powder [36]. In addition, foreign bodies are present per se in propolis, because they can be introduced by bees during the collection of resinous exudates from plants [37]. At the same time, when the beekeepers collect propolis, additional elements could be incorporated such as soil or vegetal material remains. So, the elaboration and collection of propolis impact its quality and the test for mechanic impurities or foreign bodies provides information to evaluate the importance of such contamination.
As shown in table 1, the two investigated samples of propolis comfortably meet the requirements established by the regulations of reference for ash and mechanic impurities; however, purification processes to remove mechanic impurities must be considered to obtain a raw material useful in pharmaceutics and cosmetics.
To the purpose of this study, the results obtained from waxes (n-hexane extractable material) are ofparticular concern. So, in this bee product is desirable a minor proportion of waxes, owing to the absence of active molecules in them [18]. On this basis, the investigated propolis samples must be classified as a beekeeping product of low quality considering the high percentage of these compounds quantified via the n-hexane extractable material. However, from this supposed disadvantage arises the possibility of using propolis as a raw material material for the preparation of novel pharmaceutic and cosmetic lipid systems as it will be discussed later.
On the other hand, the hive products become contaminated with heavy metals as a result of bee's foraging [38], or derivate of practices of apiculture. One of the heavy metals of higher toxicity is lead, which is mostly found in air due to pollution of men's activities. By way of example, concentrations ranging between 0.07 and 3.75 mg of lead/Kg ofpropolis were reported for Andalusian propolis [1]; and in another research, three over four samples of Argentine propolis do not comply the specification stated by the Argentine Food Code [39]. Moreover, samples of propolis from the south of Poland contains between 0.89 and 2.94 mg of lead/Kg of propolis [40].
In our case, the lead content for the two investigated propolis is lower than the USP limit test [41]. Although these results were obtained by an analytic technique less sophisticated than those reported in other research works [1, 39, 40], the USP methodology may result valid considering the objective of this research.
Regarding arsenic, low quantities were found in the investigated propolis samples (Table 1). This toxic metal in propolis is from environmental pollution coming from rocks, soil, vegetables, and diverse aquifer sources [41], and for example, concentrations between 0.075 mg/Kg and 0.130 mg/Kg are reported for Andalusian propolis [1].
It is important to consider that propolis is suggested as an environmental marker for the geographic location of its beehive [42]; therefore, the results of heavy metals can be interpreted as a positive marker for the Botanical Garden of Medellin (Antioquia) and Confines (Santander), the places of origin of the samples chosen for this work.
Quality control regarding active substances content of propolis
The test of ethanol-soluble compounds evidences the amount of resins that bees collect from several vegetable species for making propolis [43]. Thus, the higher the levels of resins are, the greatest the expected biological activity is. Accordingly, due to the eth-anol-soluble compounds are lower than the reference specifications (see table 1), the investigated samples of propolis are limited regarding the content of active substances.
With respect to oxidation index, it is a parameter for predicting the time elapsed since the propolis's harvesting, the type of storage and the antioxidant activity of the sample [44,45]. The obtained results for the worked samples shown that PrS meets the limit established in the regulations of reference; in contrast, PrJB is out-of-specification. Implications of these results can be identified from the stability of this raw material and their impact on the stability of the final pharmaceutical or cosmetic product. Thus, the oxidation index becomes one of the critical parameters to follow the quality of propolis, to define its re-test period and, to choose its packaging, storage, and transport.
Different extraction methods of biologically active compounds have been studied in the propolis [46,47], being the used dissolvent one of the more influencing factors [48,49]. Therefore, in this research work, the extracts of propolis obtained along the quality control test were used for tracking by TLC. This simple chromatographic technique allows the separation of complex mixtures and the visual detection of multiple compounds [50]. Moreover, it has been used as a quick screening for molecules with pharmacologic activity (phenolic acids and flavonoids) in different samples of propolis [51].
As shown in figure 2, there are differences in composition between the two propolis investigated and among the used extraction methods. The without-revealing plate observed at UV 254 nm (see figure 2A) evidences compounds having at least a conjugated double bond in their structure [52], for example, phenylpropane derivatives such as esters of caffeic acid, flavonoids and amino acids [53].
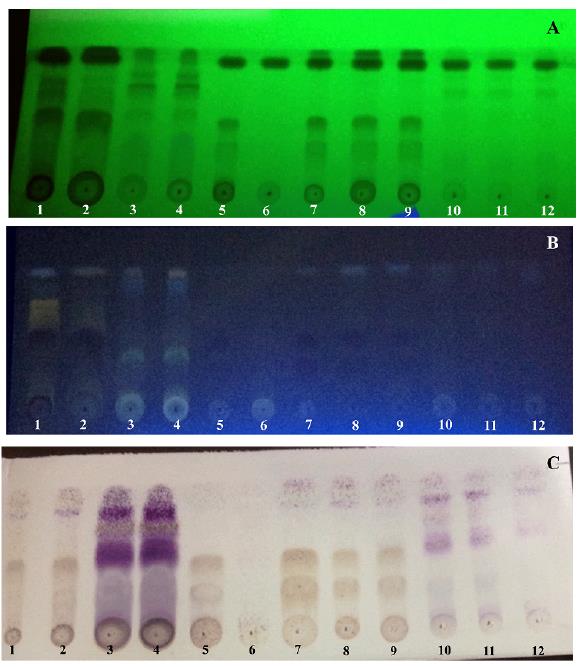
Figure 2 Tracking of propolis extracts by TLC. Mobile phase: Chloroform: EtAc (70:30). (A) Without-revealing chromatogram seen at UV 254 nm. (B) Chromatogram revealed with NP-PEG seen at UV 365 nm. (C) Chromatogram revealed with Godin. Where: 1-4: methanolic extracts for the determination of soluble residue in MeOH; 1-2: replicates from PrS; 3-4: replicates from PrJB; 5-6: ethanolic extracts after a previous extraction of waxes with n-hexane; 5: extract from PrS; 6: extract from PrJB; 7-12: ethanolic extracts for the determination of total phenols; 7-9: replicates from PrS; 10-12: replicates from PrJB.
On the other hand, NP-PEG reagent enables the detection of flavonoids, intensifying its fluorescence at UV 365 nm (see figure 2B) and whose color depends on the structure of the compounds. Aglycones are the predominant flavonoids in propolis due to enzymatic hydrolysis that bees do on the glycosidic conjugates during the collection of resins and the incorporation of waxes [54, 55]. Also, it could be a consequence of the chemical composition of the vegetable exudates, since aglycones are identified in several plants [56]. This is coherent with figure 2B, where the fluorescence at spots of application in TLC is tenuous or absent. Likewise, the use of Godin's reagent (see figure 2C) demonstrates the presence of terpenoids and phenylpropanoids [52]. Such compounds have been previously reported for propolis, including derivates of cin-namic, benzoic and phenylpropanoid acids [57-59] and volatile hydrocarbons C10 that gives it the characteristic smell [37].
To complement this work, the UV spectra of the ethanolic extracts after removal the lipid material were run because they are characteristic fingerprints for propolis of specific geographic zones that evidence differences in the composition of polyphenols and flavonoids [23, 60]. As shown in figure 3, flavonoids are detected by the presence of aromatic rings in the spectral band between 240 nm and 280 nm, that is assigned to ring A of this kind of compounds [61]. It is highlighted that a minor dilution to the recommended by Bedascarrasbure et al. [17] was required to obtain an adequate analysis (1 to 50 in EtOH for PrJB and 1 to 100 in EtOH for PrS), suggesting a low content of phenolic compounds in the investigated propolis.

Figure 3 UV spectra of ethanolic extracts of propolis from different geographic origin. A) PrS. B) PrJB.
To corroborate this latest finding, the content of total phenols was quantified according to the Folin-Ciocalteu method. Indeed, although some Colombian propolis exceed the content of phenolic compounds established for the reference standards [27, 46, 62], PrS and PrJB are out-of-specification discouraging their harnessing as an interesting source of active molecules.
In addition, considering that the types of flavonoids in the propolis are diverse [54, 57, 63] and include flavones [64], flavonols [48], flavanones [65], flavanonols [66], chalcones [67], dihydrochalcones [68], isoflavones [69], isoflavans [70,71] and neofla-vonoids [72], two reported colorimetric methods based on the formation of colored complexes (in one of them with aluminum chloride and in the other one with sodium nitride), were used to quantify the flavonoids present in the investigated samples of propolis.
It is known that when using aluminum chloride, the complexes are characterized for different absorption maxima and in some cases, the quantification around 425 nm underestimates compounds such as flavanones and their glycosides; then, the method is only useful for quantifying flavones and flavonols. Regarding the procedure using sodium nitride, it is less selective due to the formation of complexes with no-flavonoid compounds that have in their structure catecholic units as the chlorogenic acid [73].
In fact, the results for the investigated samples of propolis evidenced that flavonoids quantified by the method using sodium nitride are ten times higher than those obtained when aluminum chloride is used. For this reason, only the results based on aluminum chloride were reported in table 1 choosing the conservative estimate. Our results are lower than those previously reported by researchers in Colombia [74, 75]; but they are in line with the reported works also carried out in Colombia, where propolis does not comply with the international standards [76].
This could be attributed to the botanical sources near the hive that do not have the strong solar stimulus required to improve the synthesis of these secondary metabolites [77, 78]. Also, it must be in mind that the synthesis of flavonoids in some vegetable species depends on the several factors as the hour of day, the photoperiod and the length of the day [79], the season of the year [80, 81], the environmental stimuli and the stress abiotic factors as droughts, floods, changes of temperature and, contamination, among others [82]. In addition, the diminution in the investigated samples of the amount of phenolic molecules as flavonoids might result because of the role of propolis as a natural protection for the hive against pathogens and other agents [43].
Finally, the antioxidant activity of propolis was investigated by the TEAC method that quantifies both lipophilic and hydrophilic antioxidants [83]. The results indicate a lower content of antioxidants than other samples of propolis [76]. However, care must be taken to interpret these results because TEAC is not a specific method due to any hydroxylated molecule, without distinguishing its antioxidant ability, could also be quantified [84].
According to the last results, the investigated propolis samples do not offer advantages as a potential source of active molecules. However, low concentrations of actives, particularly antioxidant agents, may be present considering the high content of lipid compounds that might exhibit a synergist role with other compounds of the pharmaceutic and cosmetic formulations.
POTENTIALITIES OF THE INVESTIGATED PROPOLIS FOR THE DEVELOPMENT OF PHARMACEUTICAL AND COSMETIC PRODUCTS
In general terms, from the quality control standpoint, the investigated samples of Colombian propolis have a no homogeneous aspect, characteristic flavor, low content of molecules with biological activity, a high percentage of waxes and, low presence of contaminants. Consequently, different alternatives can be proposed for promoting their harnessing in the cosmetic and pharmaceutic fields. Among them, the use of propolis as starting material for developing lipid systems is highlighted.
In this way, propolis could be used for making conventional body care products containing lipids, like creams, ointments, lotions and, oily preparations, among others; e.g., propolis lipsticks have been reported [85]. However, considering their composition, the investigated propolis could be used to make innovative developments. Thus, waxes might be a key ingredient of formulations as micro and nanoparticulate lipid systems, that are continuously investigated because of their advantages associated to their entrapment efficiency for hydrophobic drugs, easy scale-up, biodegradable composition, relative nontoxic nature and stability [85-89].
Likewise, propolis can be used to prepare nanofibrous composite scaffolds [90, 91] or natural rubber latex membranes [92] for bone tissue engineering thanks to the malleable behavior that enhances the tensile strength of the polymer used as support. A green approach in the same direction has been reported by Sharaf, Higazy and Hebeish [93], who investigated the propolis applicability to obtain cotton textiles having antibacterial activity.
We know that the investigated propolis have a low content of active molecules. Nevertheless, they might be added to cosmetic formulations to contribute as a natural raw material in products with claims as antibacterial, e.g., in biocomposite films for the care of the oral cavity [94, 95], or as a moisturizer, revitalizing, and restoring of the skin's elasticity [96, 97]. In line with this, propolis can also be encapsulated in nano or microparticulate systems [88, 98] for preventing contact dermatitis or showing specific biological activity [99, 100]. By way of example, propolis loaded silver-silica systems exhibiting enhanced antifungal and antibacterial properties, have been reported. These carriers, obtained from silver nanoparticles and propolis, both located into the channels or on the surface of nanoporous supports, could be used for wound healing [87]. Also, oral pathogens can be treated by using propolis microparticles [101, 102].
It is important to bear in mind that purified propolis must be used to formulate these kinds of products attending to the concerns about safety when its use in humans is intended [15, 103]. In fact, the presence of pesticides and antibiotics, as well as the microbial quality of propolis must be taken into account. These tests were not carried out in this work for the investigated samples; so, if necessary, purification processes must be considered before their use to develop medicines and cosmetic products.
In addition, as a heterogeneous material with compounds covering all the possibilities of solubility, the formulation of homogeneous pharmaceutical forms could result in a challenge. Moreover, as it has been highlighted by Bankova [104], this chemical variability makes it difficult to get standardized propolis and the biological activity of the products, rather than their quality control, could be used as an indicative criterion for guaranteeing reproducibility in quality of the final products. Also, product designers must be aware that the industrial processing of propolis, particularly the steps involving melting of this material, could cause instabilities of the active molecules that put in risk the stability of the drug and cosmetic products containing it.
In summary, although the quality of propolis depends on several factors and it is possible to have sub-standard material due to the content of active substances, as in the case of the investigated samples, its potential use in pharmaceutics and cosmetics results attractive. Propolis could be formulated in innovative delivery systems where the mechanical properties or the presence of non-active components results advantageous. In addition, this leads to natural products according to the market trends and the consumer's requirements.
CONCLUSIONS
A comparative view of two samples of Colombian propolis was obtained from the quality control analysis carried out according to the standard methods stated by the AOAC, the USP, and procedures previously reported. The results, mainly those evidencing the low content of molecules with biological activity, reveal that these propolis are sub-standard beekeeping products. Thus, their use as a source of active molecules of interest for developing medicines and cosmetics is very limited.
Nevertheless, the high waxy content of the investigated propolis, another criterion that classifies them as sub-standard materials, open exciting potentialities for their harnessing. In this sense, taking as starting point experimental evidence of other research teams, different alternatives were proposed for promoting its application in the pharmaceutical and cosmetic industries. Among them, the use of propolis waxes as starting material for developing lipid systems is highlighted.