INTRODUCTION
In contrast with the increase in prevalence of noncommunicable diseases, infectious-contagious pathologies still remain as the primary cause of mortality in underdeveloped countries and the third cause worldwide [1]. Antimicrobial resistance (AMR) is known to increase the incidence and mortality of bacterial, fungal, parasitic, and viral infections, and thus threatens the prevention and treatment of an ever-increasing range of diseases [2-5]. Deaths from antimicrobial-resistant bacteria, for example, are predicted to rise globally from 700 000 to 10 million per year until 2050, overcoming chronic diseases such as cancer and diabetes [3]. Herein, the development of control infection policies and new antibacterial drugs are important strategies to reverse the alarming situation of AMR [5, 6].
Re(emergent) arboviruses are another serious public health problem. Five human epidemic mosquito-borne arboviruses, yellow fever virus (YFV), Dengue virus (DENV), West Nile virus (WNV), Chikungunya virus (CHIKV), and Zika virus (ZIKV), have emerged in several regions of the world in recent years [7]. Among these viruses, DENV is the most prevalent, affecting more than 400 million individuals annually in tropical and subtropical zones [8]. Currently, there is no effective and safe vaccine against the four serotypes of DENV (DENV1-4) and vector control strategies have failed. Therefore, the development of antiviral drugs that reduce viral load and disease progression are urgently necessary [9].
Thus, in terms of the development of new antimicrobial drugs, several studies have reported chalcone derivatives as promising agents [10-12]. Chalcones (1,3-diphenyl-2-propen-1-based) are naturally occurring open-chain intermediates of flavonoid and isoflavonoid biosynthesis widely distributed in plants [10]. Several studies have reported a wide range of biological activities, including anti-inflammatory, anti-gout, anti-histaminic, antitumoral, anti-obesity, anti-diabetic, anti-degenerative, and anti-spasmodic [10, 11]. In addition, the chalcone scaffold also exhibits antimicrobial effects, which target several bacteria, fungal, protozoa, and viral species of medical importance [12]. As the chalcone skeleton contains various areas for functionalization, it has been used to produce different combinations of aromatic substituents [13]. These chemical modifications might help increase biological activity, as well as improve the pharmacokinetics or toxicological properties of compounds [11]. Here, we aim to determine the antibacterial, anti-Candida, and anti-Dengue potential of new chalcone-bearing 2,4-dihydroxyl and tetrahydropyranyl moieties, previously synthetized by our group.
MATERIALS AND METHODS
Chemistry
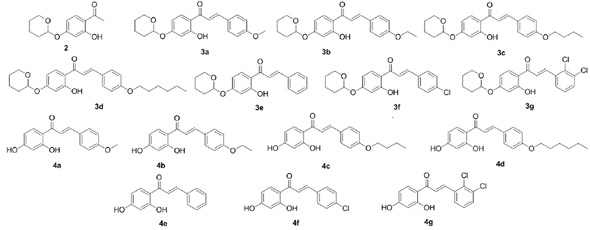
The chalcones employed in this study (figure 1) were provided by the Laboratory of Organic synthesis and Nanotechnology, Federal University of Sao Joao Del-rei, Divinópolis, MG, Brazil. These compounds were synthesized according to the methodology described by Andrade et al. [13].
Biological activity
Microorganisms and cells: All microorganisms were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA) and were kindly provided by the Reference Microorganisms Laboratory of the Oswaldo Cruz Foundation (Rio de Janeiro, Brazil). The following species were used in the antibacterial assay: Acinetobacter baumannii ATCC 19606, Klebsiella pneumoniae ATCC 43816, Enterobacter cloacae ATCC 23355, Staphylococcus aureus ATCC 29213, Staphylococcus epidermidis ATCC 12228, and Staphylococcus saprophyticus ATCC 15305. Antifungal activity was determined against five clinical isolates of Candida albicans from patients with oral candidiasis (OC), which were kindly provided by Susana Johann (Laboratory of Mycology, UFMG, Belo Horizonte, Brazil).
The cytotoxicity was determined in baby hamster kidney cells (BHK-21) (ATCC CCL-10) and anti-Dengue activity was performed using DENV serotype 2 (DENV-2) kindly donated by Erna Geessien Kroon (Laboratory of Virus, UFMG, Belo Horizonte, Brazil).
Antibacterial activity: Mueller Hinton broth (MHB; Himedia, Mumbai, India) [14] was used to determine the minimal inhibitory concentration (MIC) by broth microdilution technique according to the Clinical and Laboratory Standards Institute (CLSI) [15]. The chalcone analogs were dissolved in 50% Dimethyl sulfoxide (DMSO; Neon, São Luiz, Brazil) and serially diluted two-fold ranging from 0.49-250 µg.mL-1. The results were visualized after 24 h of incubation at 37 °C and the MIC value was considered as the lowest concentration of tested compound able to prevent visible growth. Amoxicillin (Inlab, São Luiz, Brazil) and chloramphenicol (Inlab, São Luiz, Brazil) were used as positive controls for Gram-positive and Gram-negative bacteria, respectively. Vancomycin (ABL, Sumaré, Brazil) was used as a positive control for methicillin-resistant Staphylococcus aureus (MRSA).
The minimal bactericidal concentration (MBC) was determined in accordance with Lima et al. [14], and the MIC of compound 4e in the presence of a sub-inhibitory concentration of polymyxin B (Inlab, São Luiz, Brazil) was evaluated against Gram-negative bacteria as described previously by Thangamani et al. [16]. All experiments were performed in triplicate, with at least two independent assays.
CLSI M07-A9 document [17] with modifications [14]. Briefly, 100 µL from a suspension of Candida spp. at density of 103 Colony-forming unit (CFU).mL-1, prepared in Sabouraud-dextrose broth (Himedia, Mumbai, India), was added to the wells of a microplate followed by treatment with two-fold serial dilutions (1-500 µg.mL-1) of chalcones. The microplates were then incubated at 37 °C for 48 h, and the results were read visually, with MIC (µg/mL) values defined as the lowest compound concentration without visible growth. Nystatin (Pharma Nostra, Campinas, Brazil) and ketoconazole (Sigma-Aldrich, St. Louis, USA) were employed as positive controls and DMSO was included as a solvent control at a concentration of 10% v/v. The minimum fungicidal concentration (MFC) was determined for compounds that showed fungistatic activity in the MIC assay according to methodology described by Lima et al. [14]. All data were recorded following at least three independent experiments performed in triplicate.
Cytotoxicity assay: The cytotoxicity of chalcones was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method [18]. BHK-21 cells were grown in Dulbecco's Modified Eagle Medium (DMEM; Cultilab, Campinas, Brazil) supplemented with 5% fetal bovine serum (Sigma-Aldrich, St. Louis, USA) and 0.3% penicillin-streptomycin-amphotericin solution (10,000 U.mL-1 + 10 mg.mL-1 + 2 mg.mL-1; Sigma-Aldrich, Munich, Germany). The culture was incubated at 37 °C in a humidified atmosphere containing 5% CO2. In total, 5 x 105 BHK-21 cells were added to each well of a 96-well plate and incubated for 24 h at 37 °C. Following this, cells were exposed to different dilutions of compounds (0.78200 µg/mL) and the microplates were incubated for 48 h at 37 °C. Finally, the cell viability was determined using MTT (5 mg/mL; Sigma-Aldrich, St. Louis, USA) and the cytotoxic concentration for 50% of the cells in culture (CC50) was calculated by linear regression analysis [14].
Antiviral assay: Antiviral assay was performed using confluent cultures of BHK-21 cells in 96-well plates as previously described [19]. In total, 5 x 104 BHK-21 cells were added to each well of a 96-well plate and incubated for 24 h at 37 °C. Cells and viruses were separately exposed to chalcone dilutions below the CC50 and incubated at 37 °C for 30 min. Then, cells were infected with DENV-2 at a multiplicity of infection of 0.1 and incubated for 5 days at 37 °C in a humidified atmosphere containing 5% CO2. Following incubation, the protective effect of compounds against the cytopathic action of DENV-2 in cell culture was measured using the MTT assay, as described above. Ribavirin (Sigma-Aldrich, Munich, Germany) was used as a positive control and DMSO was included as a solvent control at a concentration of 10% v/v. The effective concentration that protected 50% of the infected cells (EC50) was calculated by linear regression analysis [20] and the tests were performed in triplicate.
RESULTS AND DISCUSSION
Antibacterial activity of the chalcone derivatives (figure 1) was evaluated by determination of MICs using the broth microdilution method and by evaluation of bactericidal activity. As shown in table 1, except for compounds 4d and 4g, the 2,4-dihydroxychal-cone derivatives were active against all Staphylococcus species tested, with MICs in the range of 19.5-125 µg.mL-1. According to Mbaveng et al. [21], antimicrobial activity is considered significant when the MIC is below 20 µg.mL-1, moderate when 20 µg.mL-1 < MIC <100 µg.mL-1, and low when MIC >100 µg.mL-1. Compound 4e was the most active among the derivatives tested, showing significant antibacterial action against S. aureus (MIC 19.5 µg.mL-1), and moderate effects against S. epidermidis and S. saproticus (MIC 31.3 µg.mL-1 for both species). Compound 4e was also active against an MRSA strain (MRSA USA 300), with an MIC of 62.5 µg.mL-1 (vancomycin: MIC of 1 µg.mL-1 against MRSA USA 300; data not shown). In addition, moderate activity was also observed with compound 4c against S. aureus and S. epidermidis (MIC 62.5 µg.mL-1), and with the analogue 4b against S. aureus (MIC 62.5 µg.mL-1). The antibacterial effect of the 2,4-dihydroxychalcone series was mainly bacteriostatic, but compound 4e also demonstrated bactericidal action against methicillin-sensible and methicillin-resistant S. aureus, as it was able to kill these pathogens at 80 and 125 µg.mL-1, respectively (vancomycin: MBC 8 µg.mL-1 against MRSA USA 300; data not shown).
Table 1 Summary of bacteriostatic and bactericidal effects of chalcones derivates against selected Gram-positive and Gram-negative bacteria.
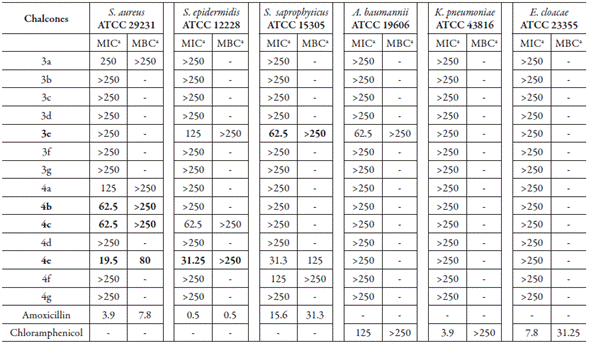
aResults were expressed in μg.mL-1 and as average of three readings. (-): No evaluated; MIC: Minimum inhibitory concentration; MBC: Minimum bactericidal Concentration. In bold is stand out the most active compound.
Tetrahydropyranyl-chalcones, in turn, were considerably less actives. This suggests that tetrahydropyranyl is not an important pharmacophoric scaffold for the antibacterial activity of chalcones. Steric factors associated with this group as well as the loss of one center receptor to hydrogen bold in relation to 2,4-dihydroxychalcones can, at least in part, explain the reduced activity of these analogs [22]. Consistent with these results, a study has shown that the addition of a tetrahydropyranyl moiety involves the loss of activity of 2-alkynoic fatty acids against S. aureus, S. saprophyticus, Bacillus cereus, Escherichia coli, K. pneumoniae, and Pseudomonas aeruginosa. However, the removal of this group is associated with high activity against all bacteria tested, including strains of MRSA [23].
In contrast to Staphylococcus spp., all Gram-negative species did not show susceptibility to the chalcone derivatives in the range of concentrations tested (0.97-250 µg.mL-1) (table 1). Herein, only the compound 3e showed activity against A. baumannii, with an MIC of 62.5 µg.mL-1. The inhibitory concentrations of the others tested compounds were greater than 250 µg.mL-1, and the MICs to chloramphenicol were 3.9, 7.8, and 125 µg.mL-1 against K. pneumoniae, E. cloacae, and A. baumannii, respectively. Thus, we have revealed that the 2,4-dihydroxychalcones are narrow-spectrum antibiotics, which overcome only Gram-positive bacteria. In accordance with this study, Silva et al. [24] showed that chalcone derivatives are active against Gram-positive bacteria (S. aureus, Bacillus subtilis, Streptococcus mutans, and Micrococcus luteus), but no activity was observed against the Gram-negative bacillus Salmonella choleraesuis, E. coli, and P. aeruginosa.
The intrinsic resistance of Gram-negative bacteria to chalcones may be related with the outer membrane (OM) of these microorganisms. In fact, many Gram-negative bacteria are particularly resistant to several antibiotics because the antibiotics cannot penetrate this barrier to reach their molecular target within the bacterial cell [25]. Interestingly, a study has shown that cationic chalcones are active not only against Gram-negative antibiotic-sensitive bacteria, but also possess high antibacterial activity against KPC2-and NDM1-producing carbapenem-resistant Enterobacterales [26]. The antibacterial activity of this group of positively charged chalcones is due to binding in the negatively charged OM of Gram-negative bacteria, and thus it is not necessary that the chalcones cross this barrier to exhibit their activity [26]. To determine the impact of the OM on the resistance of Gram-negative bacteria to the compounds in this study, we employed the test described by Thangamani et al. [16]. Following the addition of a sub-inhibitory concentration of polymyxin B in medium (0.49 µg.mL-1), the MIC of compound 4e was reduced from > 250 to 62.5 µg.mL-1 against A. baumannii (table 2), suggesting that, at least in this specie, the OM is associated with the intrinsic resistance to chalcones.
Table 2 MIC of chalcones against Gram-negative bacteria after addition of sub-inhibitory concentration of polymyxin B.
| Bacteria | MIC of Polymyxin B (µg.mL-1) | Sub-inhibitory concentration of Polymyxin B used (µg .mL-1) | Chalcone 4e (µg..mL-1) | ||
|---|---|---|---|---|---|
| Polymyxin B | |||||
| (-) | (+) | ||||
| A. baumannii ATCC 19606 | 1.95 | 0.49 | >250 | 62,5 | |
| K. pneumoniae ATCC 43816 | 1.95 | 0.49 | >250 | >250 | |
| E. c/oacae ATCC 23355 | 1.95 | 0.49 | >250 | >250 | |
To determine the antifungal potential, we selected the most active compounds between the tetrahydropyranyl-chalcones (3e) and the 2,4-dihydroxychalcones (4e) to test against oral isolates of C. albicans sensitive and resistant to conventional therapy. Compound 4e showed significant antifungal activity against isolates 3, 4, and 5 (MIC 15.6 µg.mL-1 for all) and was moderately active against strains 1 and 2 (MIC 31.3 µg.mL-1 for both) (table 3). It is also important to highlight that isolate 4 is resistant to ketoconazole, fluconazole, and itraconazole, and compound 4e is two-fold more active against this strain than the positive control (ketoconazole: MIC 31.3 µg.mL-1). In contrast with the antibacterial activity, which the chalcones were mainly bacteriostatic, the antifungal effect was characterized by good microbicidal action, with the MFC in the range of 15.6-31.3 µg.mL-1 (table 3). Compound 3e was active only against isolate 1 with an MIC of 31.3 µg.mL-1 and MFC of 62.5 µg.mL-1, thus reinforcing the notion that the tetrahydropyranyl moiety impaired the antimicrobial activity of chalcone derivatives (table 3). The anti-Candida activity of these chalcone analogs has already been determined against vaginal isolates and a reference strain of C. albicans and non-albicans, and showed good therapeutic potential in a model of vulvovaginal candidiasis (VVC) [13]. Thus, this study pointed out that, in addition to the treatment of VVC, these chalcones may also be potential agents against oral candidiasis (OC).
Table 3 Summary of fungistatic and fungicidal effects of chalcone derivates against oral isolates of Candida albicans.
| Chalconas | C. albicans Oral 1 | C. albicans Oral 2 | C. albicans Oral 3 | C. albicans Oral 4 | C. albicans Oral 5 | |||||
| MICa | MFCa | MICa | MFCa | MICa | MFCa | MICa | MFCa | MICa | MFCa | |
| 3e | 31.3 | 62.5 | 500 | >500 | 500 | >500 | 500 | >500 | 125 | >500 |
| 4e | 31.3 | 31.3 | 31.3 | 31.3 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 |
| Ketoconazole | 1 | 4 | 2 | 4 | 4 | 15.6 | 31.3 | 250 | 8 | 62.5 |
| Nystatin | 8 | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
aResults are expressed in µg.mL-1 and as average of three readings. MIC: Minimum inhibitory concentration; MFC: Minimum fungicidal Concentration. In bold is stand out the most active compound.
Antiviral activity of phenolic compounds from vegetal sources has been shown in some studies, including against emergent arboviruses such as ZIKV [27], CHIKV [28], and DENV [29]. A study showed that two cyclohexenyl chalcone (4-hydroxypanduratin A and panduratin A) derivatives from Boesenbergia rotunda (L.) roots were able to inhibit protein NS3 of DENV-2 with inhibitory constants of 21 and 25 µM, respectively, revealing the potential anti-Dengue activity of this phenolic class [30]. In this context, we investigated the anti-Dengue activity of tetrahydropyranyl-chalcones and 2,4,-dihy-droxylchalcones by assessing cellular viability. Initially, we performed the MTT assay to assess the cytotoxic effect of chalcone analogs against BHK-21 cells.
According to table 4, the CC50 value of tetrahydropyranyl-chalcones (3a-g) ranged from 13.1 to 199 µg.mL-1. Following assessing of cytotoxicity of these compounds, BHK-21 cells were treated with chalcones at concentrations lower that the CC50 of each one, and posteriorly, the cells were infected with DENV-2. The anti-Dengue effect was determined by assessing cellular viability using the MTT method as well as the cytopathic effects induced by viral multiplication. However, the chalcones employed in this study did not show activity against DENV-2 at any of the concentrations tested (table 4).
Table 4 Cytotoxic concentration (CC50, cytotoxicity assay) and effective concentration (EC50, anti-dengue assay) to 50% of cells in culture after treatment with the chalcones and infection with DENV-2.
| Chalcones | CC50 (µg.mL-1)a | EC50 ( µg.mL-1)a |
|---|---|---|
| 3a | 199.50 ± 0.57 | - |
| 3b | 17.8 ± 10.1 | - |
| 3c | 52.4 ± 32.3 | - |
| 3d | 92.1 ± 33.1 | - |
| 3e | 12.79 ± 2.65 | - |
| 3f | 13.1 ± 6.0 | - |
| 3g | 21.44 ± 2.82 | - |
| 4a | 22.73 ± 3.72 | - |
| 4b | 16.86 ± 3.99 | - |
| 4c | 10.53 ± 2.65 | - |
| 4d | 24.66 ± 4.16 | - |
| 4e | 8.67 ± 4.16 | - |
| 4f | 59.8 ± 44.4 | - |
| 4g | 45.6 ± 7.0 | - |
| Ribavirin | 23.54 ± 4.5 | 15.33 ± 2.6 |
aThe results are express as mean ± standard deviation. (-): EC50 greater than the highest concentration tested in each case.
CONCLUSION
A novel series of chalcone-bearing 2,4-dihydroxyl and tetrahydropyranyl moieties were synthesized and efficiently identified using IR, XH, and 13C NMR spectroscopy analysis. Next, the biological activity was evaluated against bacteria, Candida albicans, and DENV-2 to determine the antimicrobial effects of these compounds. Herein, our study has shown that the compound 4e (a 2,4-dihydroxylchalcone) is a potent anti-Staphyloccocci and anti-Candida agent and stands out as a promising prototype in the development of new antimicrobial drugs. The addition of a tetrahydropyranyl group to this compound (3e) was associated with lower antibacterial and antifungal activity. In contrast, Gram-negative pathogens were not susceptible to the antibacterial action of chalcones, likely because the OM of this microorganism functions as a barrier. Antiviral activity against DENV-2 was also not observed with the chalcones, which showed high cytotoxicity. In conclusion, the compound 4e (a 2,4-dihydroxylchalcone) showed a potent anti-Staphyloccocci and anti-Candida activity and stands out as a promising prototype in the development of new antimicrobial drugs. Overall, this study revealed that despite the antibacterial and antifungal activity of some 2,4-dihy-droxychalcones, additional molecular modifications will be necessary to reduce the toxicity well as increase bioavailability in Gram-negative bacteria.