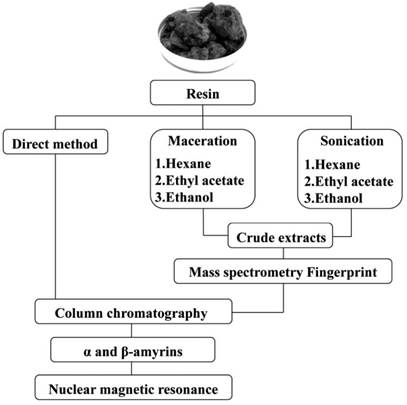
INTRODUCTION
The Amazon, with its enormous biodiversity, has a great potential of providing new products. In this context, the oleoresins produced by the Protium species (Burseraceae), commonly known as "breu branco", is very promising due to its medicinal and industrial potential [1]. This raw material is used in popular medicine as a healer, antiinflammatory, analgesic, expectorant and for ulcer treatment. It is also commonly used as an incense and natural insecticide, as well as for caulking boats. Its main industrial application is as a perfume fixative [2-4].
The oleoresin is characterized by the substantial presence of terpenoids, especially triterpenes. Triterpenes have a wide structural diversity and can be tetracyclic or pentacyclic. Subclasses oleane and ursane, the most common pentacyclic in the class of triterpenoids, and euphanus [5], the most frequent tetracyclic in Protium species, are considered chemical markers of the genus [6]. Triterpenes are constituents that have captured much interest in recent years as their pharmacological potential was discovered, which gave rise to various therapeutic activities, being used for anticancer, anti-inflammatory, antiepileptic, antiviral, antibacterial, antifungal, antidiuretic, giardicide treatments, and as an acetylcholinesterase inhibitor [7].
The major constituents of Protium resins are the pentacyclic triterpenes, alpha and beta-amyrins [2], which have been showing significant biological activity. The isolation or isomeric mixture of these substances and their derivatives have shown to be anti-inflammatory, antinociceptive, gastro- and hepatoprotective, antipruritic, anti-dementia, anxiolytic and antidepressant, hypoglycemic, and lipid-lowering agents [8-17], which have made them interesting molecules for studying drug development.
The large-scale isolation of active substances for use in pharmacological and chemical studies is an essential step toward the development of new medicines. Additionally, it is also necessary for discovering the active principle responsible for the therapeutic effect of crude extract. Several studies have been carried out to optimize extraction processes of bioactive substances. The most commonly used parameters are solvent type, extraction method, time, and temperature, according to the class of the substance of interest [18-20].
The standardization for obtaining bioactive substances on large scale allows the development of new biotechnological products for industrial application. In view of the need to find ways to improve the extraction yields of amyrins from Protium oleoresin, this work performed a factorial study aiming to optimize its isolation from commercial resins, which is considered a sustainable way for its production.
METHODOLOGY
Obtaining plant material
Samples of impure Protium resins were purchased at a traditional fair in Coari, Amazonas, Brazil. The samples were cleaned to remove impurities, such as mineral and vegetal residues, powdered in a porcelain mortar and stored in a refrigerator until the extraction procedure.
Extraction methods
The amyrin was obtained in three ways: direct method, using the crude resin fractioned directly in chromatographic column; maceration and sonication both using hexane, ethyl acetate, and ethanol (Labsynth, São Paulo, Brazil) as eluents. Then, all extracts obtained were fractioned by chromatographic column. All procedures were carried out in sextuplicate (figure 1).
Obtainment and fractionation of the crude extracts
Cold maceration was carried out for a period of 72 h at a 1:10 (m/v) resin and extractive solvent ratio. After the extraction time, the material was subjected to simple filtration and subsequent removal of the solvent in a rotary evaporator under reduced pressure. After removing the solvent, the extract was fractionated in a chromatographic column.
Sonication extraction was performed on an ultrasound device (Ultrasonic Cleaner, Unique) for 20 min at a 1:10 (m/v) ratio of resin and extractive solvent. After the extraction time, the solution was subjected to filtration and evaporation, in the same manner as the previous methodology.
Chromatographic separation was performed in a glass column with a 2 cm internal diameter. One (1) cm of cotton was placed on the bottom base of the column. Then, 20 cm of silica gel (Silicycle G60 70-230 mesh) in height was added to the column and used as stationary phase, eluted with a 200 mL gradient of hexane and ethyl acetate at ratios: 95:5; 90:10; 80:20; 70:30; 60:40; 1:1 as mobile phase. The eluting was observed under ultraviolet light (2254). The collected fractions (50 mL) were grouped according to the similarity observed by thin layer chromatography (TLC). Subsequently, the amyrin mixture was washed with acetone and methanol and the yield was calculated.
Direct extraction
The powdered resin (30 g) was subjected to column chromatography using silica gel as stationary phase and combinations of hexane, ethyl acetate, and methanol as mobile phases, as previously described. Eight (8) fractions were obtained. After solvent removal, the fractions were grouped according to the similarity in TLC for calculating the yield.
Electrospray ionization mass spectrometry fingerprint
All extracts were dissolved in methanol and ethyl acetate (J. T. Baker, Thermo Fisher Scientific, Massachusetts, USA) and analyzed by direct electrospray ionization mass spectrometry (ESI-MS) on an ion-trap mass spectrometer (LCQ Fleet, Thermo Fisher Scientific, Massachusetts, USA). The MS spectra were acquired at a mass/charge (m/z) range from 100 to 1000. Tentative identifications were performed by manual interpretation of the mass spectra.
Nuclear magnetic resonance (NMR)
Nuclear Magnetic Resonance 1H and 13C spectra were obtained on a 300 MHz equipment (Fourier 300 model, Bruker, Massachusetts, USA), using 550 µL of chloroform-d (Acros Organics, Massachusetts, USA) in a tube of 5 mm to solubilize 15 mg of sample.
Statistical analysis
The effect related to the type of extraction in the alpha and beta-amyrins yielding was evaluated by a factor analysis, considering: the extraction method (factor A), which varied in two levels (maceration and sonication); and the solvent (factor B), which varied in three levels (hexane, ethyl acetate, and ethanol). Data was presented in mean ± standard deviation and compared by analysis of variance (ANOVA) followed by the Tukey test, considering p<0.05 as statistically significant.
RESULTS AND DISCUSSION
Several methodologies are described for the preparation of extracts from plants, microorganisms or even animals (sponges) aiming at the isolation of their chemical constituents, as well as for obtaining them on a larger scale. Thus, different extractive methods and solvents were used to obtain the major constituents, alpha and beta-amyrins, in order to develop a process for large-scale obtainment and improved cost effectiveness.
Six yield measures (%) were obtained in each treatment or level of the experiment (table 1). The figure below shows the box graph (box plot) for each level of the variable yield in the two extraction types (maceration and sonication) with the three solvent types (figure 2A and 2B) and the correlation of the two extraction methods. A strong correlation between the type of solvent and yield was found (figure 2C).
Table 1 Yield of extracts obtained by different extraction methods.
| Solvent | Extraction method | Yield (%) |
|---|---|---|
| Hexane | Maceration | 21.43 ± 0.79b |
| Sonication | 17.33 ± 0.80e | |
| Ethyl acetate | Maceration | 79.17 ± 1.89a |
| Sonication | 76.98 ± 1.49d | |
| Ethanol | Maceration | 79.03 ± 1.67a |
| Sonication | 44.96 ± 0.62c |
a,b,c,d interactions with the same letters do not significantly differ from one other at the 5% level of significance.
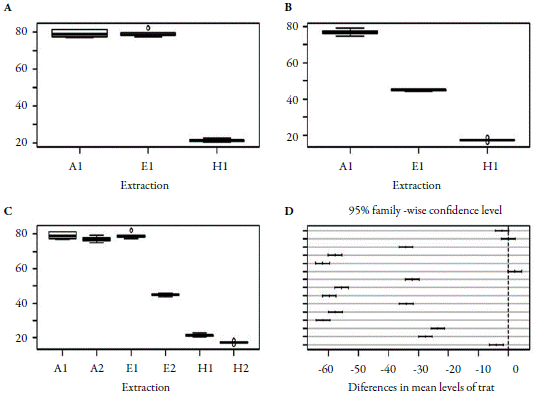
Figure 2 Analysis of the variance of yields in different extractions: (A) maceration (A1, E1 and H1); (B) sonication (A2, E2 and H2); (C) both methods; (D) differences in mean levels of methods. A1 and A2- ethyl acetate; E1 and E2- ethanol; H1 and H2-hexane.
The same delineation was used to evaluate the fraction yield of the columns where the substance of interest was identified. Then, the treatment with the best amyrin isolation yield was compared to the yield obtained through fractionation of the resin in column without any previous treatment.
By analyzing the yield of the substances obtained after the solvent extraction, a higher yield of pure substance was observed using solvent hexane as shown in table 2. The analysis of variance for the yield obtained in the columns showed that only the type of solvent significantly (p < 0.05) affects the resin yield in the identification of the substance of interest.
Table 2 Yield of alpha and beta-amyrins mixture according to the type of solvent.
| Solvent | Average yield (%) |
|---|---|
| Hexane | 37.91 ± 5.35a |
| Ethyl acetate | 26.50 ± 6.70b |
| Ethanol | 21.59 ± 4.81b |
a,bFor different letters, the treatments differ significantly from one other at the 5% level obtained by the Tukey test.
As the highest yield percentage was obtained by using solvent hexane, a test comparing the means of the maceration and sonication methods with this solvent was conducted, and the yield was obtained in the columns of pure resin. By means of this comparison, no significant difference was verified between the yield obtained in the columns using hexane and that using pure resin, as observed in table 3.
Table 3 Yield of alpha and beta-amyrins according to the type of extraction using hexane.
| Extraction method | Yield (%) |
|---|---|
| Maceration | 38.16 ± 2.06a |
| Sonication | 37.67 ± 8.21a |
| Direct method | 32.05 ± 2.40a |
aFordifferent letters, treatments differ significantly at the 5% level obtained by the Tukey test.
The statistical analysis of the extract yields showed that the type of solvent, the extraction method and the solvent interaction versus extraction method significantly <0.05) affected the resin yield in the extracts, allowing the ideal extraction method to be chosen, as well as the solvent.
Ethyl acetate extracts showed higher extraction yields and, when purified by column chromatography, the fractions obtained with hexane as solvent showed a higher content of alpha and beta-amyrins isolated in fractions F3 and F4. The direct method presented a 34% yield; however, the major substance did not have the purity of the fractions from the other extracts, requiring further treatment. It is noteworthy that in previous studies with the Protium heptaphyllum resin, the yield of the alpha and beta-amyrins mixture was about 3% [4], while that from 20 g resin obtained a 450 mg of alpha and beta-amyrins mixture [21, 22].
All the chromatographic columns made with the extracts, as well as the direct extraction of the resin in natura, were accompanied by TLC, which made it possible to observe the retention factor (Rf) of alpha and beta-amyrins, compared to the commercial standard in elution with hexane /ethyl acetate (9:1) (Rf= 0.41) (figure 3).
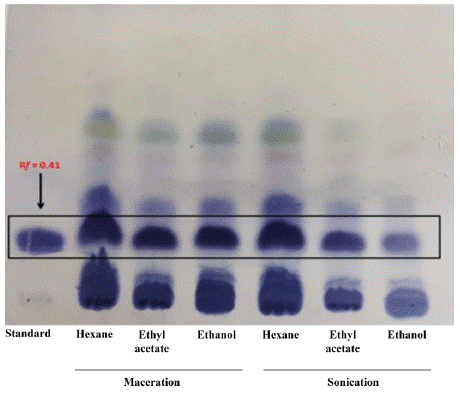
Figure 3 TLC of the extracts obtained by the maceration and sonication processes in comparison to the amyrin standards.
Although the yield of the isolates in maceration and sonication techniques presented higher values (38 and 37% respectively) than direct extraction (32%), it is observed that the use of direct extraction presents greater advantages since it does not need pre-processes with extraction techniques to obtain the enriched extract. Thus, direct extraction becomes an important method of obtaining these substances.
Similar results were also observed from the extraction of amirin from latex of Calotropis gigantea using the Soxhlet and batch extraction methods, in which it was observed that the best solvent for extraction using these methods are those of high polarity, methanol and ethanol [23]. However, the yield of the alpha and beta-amyrins mixture obtained in this work was higher.
Analysis by mass spectrometry fingerprint showed ions of m/z 409 corresponding to alpha and beta-amyrins, which are the major ions in the positive mode ESI-MS spectra of all extracts. This ion peak corresponds to a molecular ion of amyrins with the loss of a water molecule (figure 4).
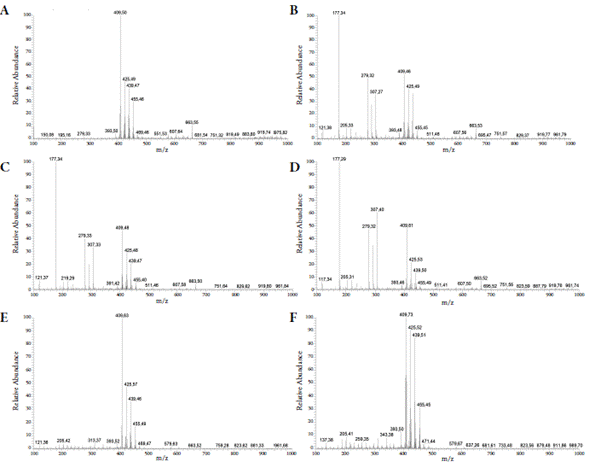
Figure 4 Mass spectrometry fingerprint. Hexane extract: (A) maceration and (B) sonication. Ethyl acetate extract: (C) maceration and (D) soni cation. Ethanol extract: (E) maceration and (F) sonication.
The 425 and 439 m/z ions were identified as being lupenone and lupenone-derived oxime and such substances have already been reported in resins of species of Protium sp. There was also the presence of ions 121 and 177 m/z, which are characteristic signals of contaminants known as phthalic acid esters.
The presence of alpha and beta-amirin, as well as lupenone and lupenone-derived oxime have already been reported in literature, both in the resin and in the leaves of P. heptaphyllum, corroborating the results of the mass spectrometry analysis of the different resin extracts found in our study [24, 25].
The presence of the mass signals characteristic of phytallic acid derivatives was also observed, however these are considered one of the most known contaminants in spectrometric analysis, derived from the solvent's contact with plastic [26-28]. Thus, they were not considered compounds present in the resin.
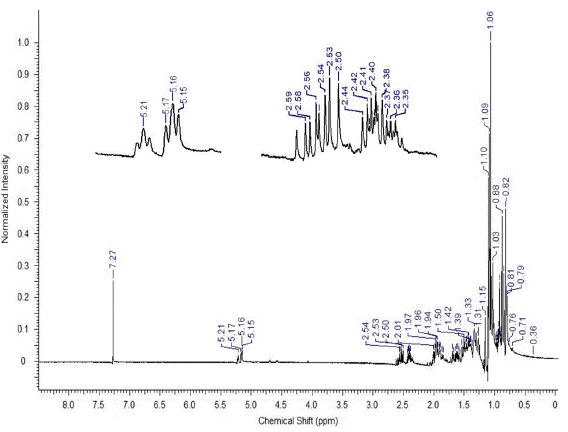
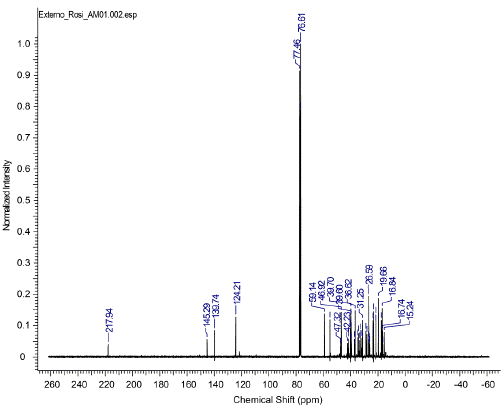
The structure of the triterpenic mixture of alpha and beta-amyrins was confirmed by NMR analysis. Although there are some signs of chemical shifts in the NMR of 1H and 13C when compared with the literature data, the structural elucidation presented in this work is unambiguous, since there are many studies in literature discussing the typical shifts presented by these structures, which was clearly detected in our spectra [7, 21, 22, 25, 29].
Analysis of 1H and 13C in nuclear magnetic resonance spectra showed characteristic signals of the oleane skeleton. Doublets corresponding to carbinolic carbons, which are characteristic of triterpenoids of 3-beta-OH type and two triplets in S 5.16 and 5.21, correspond to the CH bond of olefins. In the 13C NMR spectra, 30 characteristic signals were found: signals of non-hydrogenated sp2 carbon (C-13) at S 145.29 and C-12 carbon at δ 121.5 ppm, which corresponded to the double bond between the beta-amyrin carbons, in addition to the displacements at 124.20 and 139.74, referring to the double bond of alpha-amyrin C-12 and C-13 carbons, characteristic of the triterpenic mixture of alpha and beta amyrin (figures A1 and A2 of Appendix) [30].
Thus, this study was able to optimize the extractive process for obtaining the alpha and beta-amyrins mixture from Protium sp oleoresin and opens the perspective for the large-scale isolation of amyrins for future industrial use and pharmacological testing of these substances individually. In addition, using these isolates for chemical synthesis and structural adjustments according to purpose becomes feasible, whether for the cosmetic or pharmaceutical industry.