INTRODUCTION
Bixa orellana L. is a plant native to Brazil, but it is also present in other tropical countries such as Peru, Columbia, Ecuador, Mexico, Indonesia, India and East Africa [1-3]. It is popularly named as "Urucum" in Brazil and "Annatto in United States of America [2]. B. orellana is a tree that has between 3 and 10 meters of height, being more often found of 3 to 5 meters, has the thin trunk with approximately 30 cm of diameter with dark coloration. Its leaves have on average 15 cm in length, with 5 to 10 cm of width, fine with green coloration [4, 5].
The seeds measure around 0.3 to 0.5 cm in length with an approximate diameter of 0.3 cm and often they have a conical shape with an elongated base [5, 6]. Seeds are rich in carotenoids and are source of a natural dye with low toxicity [7, 8]. In this sense, the seeds are considered the most important commercial part of the plant due their industrial applications for production of fabrics and cosmetics [5, 9].
B. orellana is widely used in folk medicine to treat different pathologies. For example, the tea seeds are often used as a laxative, cardiac tonic, hypotensive, expectorant, antimicrobial agent and sometimes used as an anti-inflammatory formulation for treatment of pulmonary diseases such as bronchitis. Products derived from seeds is also used as eye drops for redness [2]. Its leaves are used for treatment of snakebite [10], diarrhea, gonorrhea, [11], hepatitis [2], gastritis [12], diuretic, antipyretic, and for skin problems [2]. These reports have encouraged other studies to provide scientific evidences to the ethnopharmacological knowledge and also in order to identify and isolate the active(s) substance(s).
Herein, we provided a review about the compounds with antimicrobial activity obtained from B. orellana. The articles were searched on Pubmed database (1998 to March 2019) scientific articles. Search terms were: 'B. orellana', 'antimicrobial activity' and 'antibacterial'. An exhaustive literature search was performed, but only urucum extracts and isolated compounds with positive results were included. This review was made due to the recent increase in published articles regarding B. orellana, as well as the increase in the number of publications in this regard.
COMPOUNDS OF SEED WITH ANTIMICROBIAL ACTIVITY
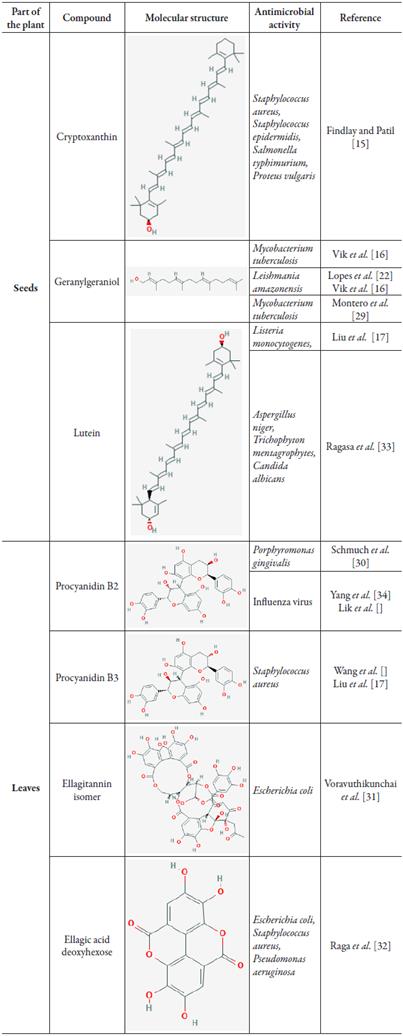
The phytochemical compounds of the B. orellana seeds were described in the literature by Galindo-Cuspinera and Rankin (2005) [13]. After the discovery of these compounds they were studied for their antimicrobial activity [14-18]. The compounds of B. orellana seeds that exhibit some antimicrobial activity according to the literature are described below. The chemical structure of the compounds can be visualized in table 1.
COMPOUNDS OF LEAVES WITH ANTIMICROBIAL ACTIVITY
The phytochemical profile of the leaves of B. Orellana was evaluated and it presented the following compounds: Procyanidin B-2, Procyanidin B-3, Granatin B, Neostrictinin, Ellagirannin isomer, kaempferol-3-O-/3-D-6-β-coumaroyl) glucopyranoside, ellagic acid glucoside, kaempferol-3-O-D-glucoside, ellagic acid deoxyhexose [19]. The following are listed which of these compounds present antimicrobial activity according to the literature. The chemical structure of the compounds can be visualized in table 1.
Crude extract
Leishmaniasis is a neglected tropical disease caused by Leishmania protozoa. There is currently no vaccine against leishmaniasis, and chemotherapy remains the only effective control. Some research on plants has shown a successful approach to obtain new antileishmanial alternatives. Herein, the in vitro and in vivo effects of the essential oil from B. orellana seeds against Leishmania amazonensis were evaluated. The oil showed activity against intracellular amastigote form (IC50 = 8.5 µg/mL), while the cytotoxic concentration was sevenfold higher for the host cells. The ability of Bixa oil to control disease progression of established cutaneous leishmaniasis in BALB/c mice was demonstrated, after a treatment with 30 mg/kg by intraperitoneal administration over 14 days [20]. Other study performed with Leishmania amazonenses was demonstrated similar results[21].
Geranylgeraniol
Lopes et al., (2012) demonstrated the activity of geranylgeraniol, the major bioactive constituent from seeds of B. orellana, against L. amazonensis. Geranylgeraniol was identified through (1)H and (13)C nuclear magnetic resonance imaging and DEPT. The compound inhibited the promastigote and intracellular amastigote forms, with IC (50) of 11 ± 1.0 and 17.5 ± 0.7 [zg/mL, respectively. This compound was also more toxic to parasites than to macrophages and did not cause lysis in human blood cells [22].
Activity against bacteria
Several studies provide scientific evidences about the action of B. orellana crude extracts against different bacteria. These extracts were obtained from both seeds [2, 23-26] and leaves [27], against bacteria of different species. Among the species of bacteria described in the literature are: methicillin-resistant Staphylococcus aureus (MRSA) [23, 25, 27]; Bacillus cereus [24, 25]; Escherichia coli [24, 27]; Streptococcus thermophilus, Lactobacillus casei subsp. casei, Lactococcus lactis, Paenibacillus polymyxa; Clostridium perfringens [25]; Bacillus subtilis; Streptococcus pyogenes; Salmonella typhi; Pseudomonas aeroginosa [27]; Mycobacterium abscessus subsp. massiliense [19].
Crude extract
As mentioned above, the seeds of B. orellana is used to prepare tea and infusions for treatment of infections due their antimicrobial and anti-inflammatory activities [2]. In this sense, crude extracts and essential oils were targets of some studies to prove their pharmacological potential. The hydroalcoholic extract of B. orellana seeds was able to inhibit the growth of bacteria: S. thermophilus, Lactobacillus casei subsp. casei, L. lactis, and P. polymyxa, microorganisms of significance in food fermentation, food preservation, and human health [25].
Another research has also demonstrated the activity of the essential oil of B. orellana against other species of pathogenic bacteria. In this work the MIC of the hidroal-colic extract was evaluated, the different bacteria and their respective MICs are listed below: B. cereus (MIC: 0.08 [µg/mL); C. perfringens (MIC: 0.31 [µg/mL) [24]. In other study carried out in Nigeria, the antimicrobial activity of extract of B. orellana seeds and Piper Guinean fruit extract was evaluated, demonstrating antimicrobial activity against Pseudomonas aeruginosa UCH 655 strain at 5 mg/mL [26].
Research developed in vitro evaluating the antimicrobial activity of medicinal plants used in Colombia, showed the activity of the hydroalcoholic extract of seeds ofB. orellana against bacteria causing non-nosocomial infections. These results demonstrate a minimum inhibitory concentration (MIC) was 0.8 µg/mL against E. coli and also a MIC was 0.2 µg/mL against B. cereus, which were lower than the values found for other plants tested in this study [24].
The high antimicrobial potential of extracts obtained from B. orellana promoted other research in order to endorse the biotechnological applications of them. An interesting work demonstrated B. orellana seeds powder could maintain the physicochemical properties of pork patties. This effect was related to the antioxidant and antimicrobial abilities of B. orellana seeds. The authors reported that the meat exposed to B. orellana seeds powder lower microbial counts for Enterobacteriaceae than control group. Therefore, B. orellana seed powder might be a good source of natural antioxidants for the production of meat products [28].
In other study, with tocotrienols from hydroalcoholic extract of B. orellana seeds was shown as an effective agent against MRSA in experimental infection model using BALB/c mice. In this work the bacteria were inoculated by subcutaneous injection, at a concentration of 5x106 CFU per animal. The results demonstrate a significant delay in the growth of MRSA in mice treated with B. orellana seed extract in contrast to the infected and untreated group [23]. The authors also reported the extract improved the in vivo activity of daptomycin in the treatment of MRSA infection [23].
The hydroalcoholic extract (BoHE) and the ethyl acetate fraction (BoEA) from B. orellana leaves also showed activity against pathogenic bacteria [19, 27]. The action of leaves extracts against infection by fast growing mycobacteria (RGM), specifically Mycobacterium abscessus subsp. massiliense. The MIC of the extract against M. abscessus was obtained resulting in 2.34 mg/mL for BoHE and 0.39 mg/mL for BoEA. When comparing the cytotoxicity also evaluated in this work, the result of the Selective Index of 19.8 for BoEA and 3.2 for BoHE was obtained. This shows an important antimicrobial activity for this mycobacterium [19].
Other studies using the ethanolic extract of the leaves of B. orellana were carried out with the purpose of evaluating the antimicrobial activity through observation of the colonies growth. These works demonstrated good activity of the extract against the bacteria: B. subtilis, S. aureus, S. pyogenes, S. tythi, P. aeruginosa and E. coli [27].
B-cryptoxanthin
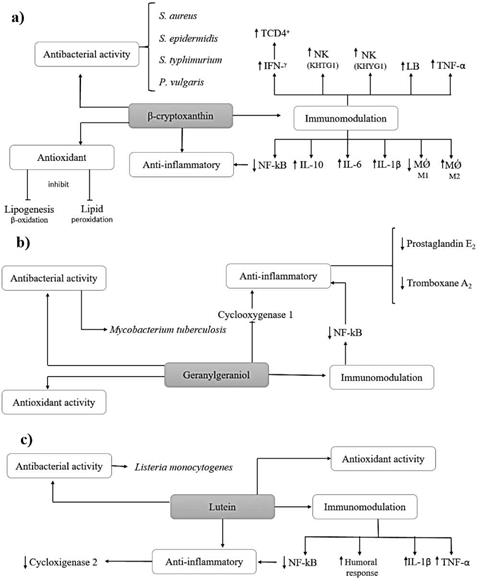
Cryptoxanthin is not a unique compound of B. orellana seeds, it can also be found in other plant species, such as Navicula delognei L. [15]. The main activities of β-cryptoxanthin as described in figure 1a. The evaluations of the antimicrobial activity of cryptoxanthin from N. delognei were performed by the same author, and demonstrated an important antibacterial activity against Staphylococcus aureus, Staphylococcus epidermidis, Salmonella typhimurium, and Proteus vulgaris through the evaluation of zone of inhibition [15].
Geranylgeraniol
Geranylgeraniol is a compound of the terpenes family. This was evaluated for its antimicrobial activity against Mycobacterium tuberculosis in vitro. For this, their capacity to inhibit pathogen growth was evaluated, which resulted in a MIC of1.56 µg/mL, showing its capacity as a growth inhibitor of M. tuberculosis [16]. Other studies shown that geranylgeraniol regulates negatively caspase-1 implication in the Th1 response against Mycobacterium tuberculosis [29].
Lutein
Lutein is a small natural molecule present in fruits and vegetables that demonstrates good activity as an inhibitor of listeriolysin-O-oligomerization (LLO), an important virulence factor of the bacteria Listeria monocytogenes. It is in vitro and in vivo activity against L. monocytogenes bacteria was evaluated. The results demonstrate a significant reduction in bacterial growth as well as an important specificity for the bacteria, showing no toxicity to BALB/c mice [17].
Procyanidin B2
Procyanidin B2 is not a compost exclusive of B. orellana leaves, it is also present in other plant species, such as Rumex acetosa L. The antimicrobial activity of procyanidin B2 of R. acetosa was evaluated against Porphyromonas gingivalis, an important pathogen causing gingivitis as well as other systemic infections. The results demonstrate a bacteriostatic effect against the colonization of this bacterium, as well as the loss of the adhesion capacity of the bacteria in vitro [30].
Procyanidin B3
Procyanidin B3 is not a compound exclusive of B. orellana leaves. It is also present in the flowers of Rhododendron formosanum. Its bactericidal activity was evaluated against S. aureus infections. Their results showed a MIC of 4 µg/mL, in addition to an important antioxidant activity [17].
Ellagitannin isomer
Ellagitannin isomer is a compound present in several plant species, among them B. orellana leaves [19] and Quercus infectoria [31]. The antimicrobial activity of ellagitannin isomer was evaluated in mice infected with E. coli. The results of this study demonstrate an important reduction of the microbial load in comparison with the control group, as well as activity as renal protector [31].
Ellagic acid deoxyhexose
Ellagic acid deoxyhexose is a sesquiterpenes present in leaves of B. orellana that has activities of gastric, analgesic, hypoglycemic and antimicrobial protector, being proven to inhibit the growth of E. coli; S. aureus and P. aeruginosa [32].
Lutein
Blumea balsamifera leaves similar to B. orellana seeds also have Lutein in their composition. In evaluating the antifungal activity of B. balsamifera compounds, the fungistatic action of lutein resulted in low growth of Aspergillus niger, Trichophyton mentagrophytes, and Candida albicans [33].
Procyanidin B2
Influenza poses a particular risk of severe outcomes in the elderly, the very young and those with underlying diseases. Tea polyphenols are the natural phenolic compounds in teas, and principally consist of catechins, proanthocyanidins, flavonols, and the aflavins, which antiviral activities have been reported recently. Some studies shown a significantly damage in viral structures and antiviral activity of procyanidin B2 [34, 35].
Lutein
Despite the availability of an effective vaccine, the hepatitis B virus (HBV) infection and its treatment remains one of the foremost public health problems in the world. The anti-HBV activity of lutein in vitro was investigated. The antiviral activity of lutein was examined by detecting the levels of HBsAg, HBeAg and extracellular HBV DNA in stable HBV-producing human hepatoblastoma HepG2 2.2.15 cells. It was found that lutein effectively suppressed the secretion of HBsAg from HepG2 2.2.15 cells in a dose-dependent manner, and it also suppressed the amount of extracellular HBV DNA [36].
Crude extract
Some studies shown an important anti-inflammatory activity against acute and chronic inflammations in rats. Acute inflammatory reactions induced in rats paws by carrageenan, histamine, serotonin, and bradykinin were all inhibited by the oral administration of 50 and 150 mg/Kg of B. orellana leaves extract. In the same study chronic inflammation was induced and treated with B. orellana extracts when administered 150 mg/Kg of extract [25]. It was confirmed four years later by Keong et al. [37], in their research was evaluated the anti-inflammatory effects of B. orellana in rats with edema induced by bradykynin. In that study Bixa leaf extract shown a significantly suppressed the inflammatory markers. The extract also decreased nitric oxide production, indicating that the anti-inflammatory effect may be related to a reduction in reactive oxygen species [37, 38]. The antioxidant and free radical scavenging activities of extracts of Bixa leaves were also demonstrated by several authors [38, 39].
Yong et al. evaluated the anti-inflammatory activities of aqueous extract of B. orellana leaves and its possible mechanisms in animal models. The anti-inflamatory activity of the extract was evaluated using serotonin-induced rat edema, increased peritoneal vascular permeability, and evaluation of leukocyte infiltrations. Was evaluated the nitric oxide (NO) production, as well the vascular growth endothelial growth factor (VEGF). That research showed the anti-inflammatory activity of B. orellana extract, indicated by the suppression of increased vascular permeability and leukocyte infiltration. The authors concluded the inhibition of these inflammatory events are probably mediated via inhibition of NO and VEGF formation and release [40]
B-cryptoxanthin
The anti-inflammatory activity from cryptoxanthin was evaluated in mice with nonalcoholic steatohepatitis. That study evaluated the anti-inflammatory effects on lipopoly-saccharide (LPS)-induced inflammation in mice. The results showed that significantly inhibited LPS-induced decreases in cell viability and in the percentage of apoptotic cells. The same study shown an up-regulation of tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), interleukin-6 (IL-6) and interleukin-1β (IL-1β) in Sertoli cells, and down-regulation of NF-kB resulting in suppression of inflammation [17].
Other studies shown an immunomodulation action, being a significantly increase IFN-g production and NK cytotoxic activity in human KHTG-1 natural killers (NK) cells [41] and KHYG-1 NK cells [42]. Studies performed in rabbits shown an important increase to CD4+ lymphocytes count and humoral immunity [43].
The antioxidant activity of β-cryptoxanthin was evaluated in non-alcoholic fatty liver disease by several authors [44-47]. That's studies shown an important reduction in lipogenesis β-oxidation [48-50], and reduction in lipid peroxidation [48, 51] showing a protective response in steatohepatitis and cirrhosis. In addition that compound shown an influence in macrophage polarization, increasing the polarization for macrophages M2, and reducing the polarization for macrophages M1 [9]. For more information on the antioxidant activity of β-cryptoxanthin we recommended the review of Ni et al. [17].
Geranylgeraniol
The anti-inflammatory activity of geranylgeraniol was evaluated by Giriwono et al. In that study rats were fed a diet supplemented with or without geranylgeraniol for 10 days. Then, these animals were submitted to intraperitoneal injection of 0.5 mg/Kg LPS or vehicle. The results shown that animals with supplementation demonstrated a significantly suppression of NF-kB in liver. The authors concluded the supplementation with geranylgeraniol was sufficient to inhibit inflammation in liver [52, 53].
In other study, geranylgeraniol isolated from fruit oil of Pterodon pubescens was evaluated in rabbits. That shown a significantly inhibition of platelets aggregation induced by U-46619 and thrombin, associated with the fruit oil and geranylgeraniol suppression of prostaglandin E2 and thromboxane A2. That demonstrate the geranylgeraniol was involved in the metabolization by inhibiting the cyclooxygenase enzyme [54]. In a study performed by Silva et al., the evaluation of efficacy of encapsulated geranylgeraniol shown an important antioxidant activity [55]. The main activities of geranylgeraniol are described in figure 1b.
Lutein
In a study performed by Karakurt et al., rats with induced retinal injury was submitted by treatment with lutein. In that study was observed a statistical significantly higher (p<0.001) reduction of IL-1β and TNF-α levels in animals submitted by lutein, in addition was observed the antioxidant activity [56, 57]. Some studies shown an anti-oxidant activity[58-60]. Other several studies shown that lutein was an important anti-inflammatory activity [58, 60-62].
Tan et al. investigated the effect of lutein against severe traumatic brain injury (STBI) and examined the mechanism of this protective effect. In that study was observed that a treatment with lutein effectively down-regulated the expression of NF-kB p65 and cyclooxygenase-2 (COX-2), intercellular adhesion molecule (ICAM)-1 protein, and upregulated nuclear factor erythroid 2 like 2 (Nrf-2) and endothelin-1 protein levels in STBI rats. These findings demonstrated that lutein protects against STBI, it has anti-inflammation and antioxidative effects and alters ICAM-1/Nrf-2 expression, which may be a novel therapeutic for STBI the clinic [18].
Other study evaluated the effects of lutein and conjugated linoleic acid (CLA) on growth performance and immune response of broiler chickens were evaluated in the presence and absence of Salmonella lipopolysaccharide (LPS) immune challenge. That study shown that CLA decreased broiler chicken growth performance, but lutein could prevent this negative effect (depending on CLA dose). Lutein had an anti-inflammatory effect, and a 2% CLA supplementation improved the humoral immune response [63]. The main activities of geranylgeraniol were described in figure 1c.
CONCLUSION
Bixa orellana is a plant present in Latin America, mainly in tropical regions, with important antimicrobial capacity. It possesses activity against both gram-negative and gram-positive bacteria. In addition, to activity against BAAR bacteria such as M. tuberculosis, M. abscessus and human pathogenic bacteria. Therefore, it is an important target of future studies, since it can represent an important advance in the treatment of bacterial infections. This becomes important as it opens up the possibility of treating bacteria resistant to commercial antibiotics. We believe that this review work may help in the understanding and incentive of new research for antimicrobial discoveries using different B. orellana compounds. Facilitating scientific development.