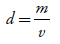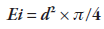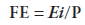INTRODUCTION
Phytotherapy is an ancient practice that uses plants to treat diseases, being an alternative that does not use isolated active substances, preserving the original composition of the original plant [1, 2]. It is still possible to notice a certain resistance from prescribing professionals to the use of medicinal plants and herbal medicines. This low adherence occurs, often, due to the discredit in therapy and the lack of technical-scientific knowledge [3, 4].
Due to the high consumption of medicinal plants/herbal medicines and insecurity about their quality controls, on June 22, 2006, the National Policy on Medicinal Plants and Herbal Medicines [5] was created in Brazil, through a decree of the presidential republic. This measure is of great importance for SUS, which aims in its guidelines to ensure the effectiveness, quality and safety of these products, in addition to easy access for treatment. It is also the aim of SUS that these treatments be a less costly alternative, especially for poor communities.
Currently, the phytotherapy is experiencing a new level with recent publications, bringing credibility and security to patients and health professionals. Thus, transforming it not only in an alternative therapy, but in a possibility that the herbal medicines, in some cases, are considered as treatment of first choice [4].
Skin lesions, burns, skin infections and chronic wounds, have a greater difficulty in healing, impair the functions of the skin, and in more severe cases can lead to death. Thus, they require greater care in the long term, increasing costs for health systems worldwide [6].
In order to minimize costs, the use of medicinal plants and natural products is being a new alternative for the treatment of wounds, especially in developing countries [7]. The search for alternative therapies to promote wound healing has been intensified, while modern therapies with antibiotics and corticosteroids have been neglected due to the side effects that conventional drugs can cause [8].
Aloe vera L. or Aloe barbadensis, popularly known as "babosa", is one of the most important medicinal plants in the treatment of wounds, considering that countless studies report its healing and anti-inflammatory properties [9].
Considering the high consumption of medicinal plants and natural products as low-cost treatments by the low-income population, and understanding that the lack of uniformity of the chemical composition, together with the presence of contaminants, cause deficiency in the safety of these products, it is important to develop formulations that ensure the quality of medicinal products of plant origin, since it directly interferes with efficacy and may offer risks to consumer health.
In this context, the present study aimed to develop and characterize a formulation of aloe gel as an alternative for the treatment of wounds, as well as to compose the portfolio of formulations of the Pharmacy School Manuel Casado de Almeida of the Federal University of Campina Grande, Education Center and Health, Campus Cuité (PB).
MATERIAL AND METHODS
The research was carried out at the Federal University of Campina Grande, campus Cuité (CES), in the teaching laboratories of the Pharmacy course and in the Pharmacy School Manoel Casado de Almeida.
Plant material
Initially, Aloe vera leaves were collected from the medicinal plant garden at the Education and Health Center (CES), and then the material was prepared, which included: washing, removing mucilage and crushing to prepare the glycolic extract.
Preparation of glycolic extract
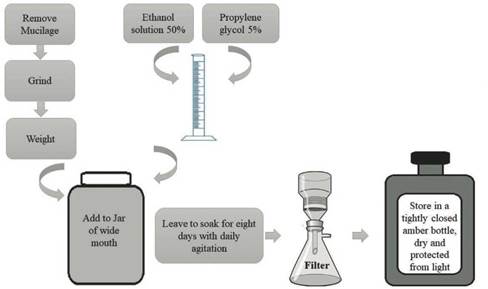
The extract was prepared according to the steps shown in figure 1. After crushing, the material was weighed and added in a wide-mouthed bottle. Separately, in a beaker, a solution of 50% Ethanol and 5% Propylene Glycol was prepared, and then added to the flask containing the mucilage, which was left to soak for eight days with daily stirring. After this period, the material was filtered, stored in amber glass, in a dry place and protected from light [10].
Preparation of Aloe vera gel
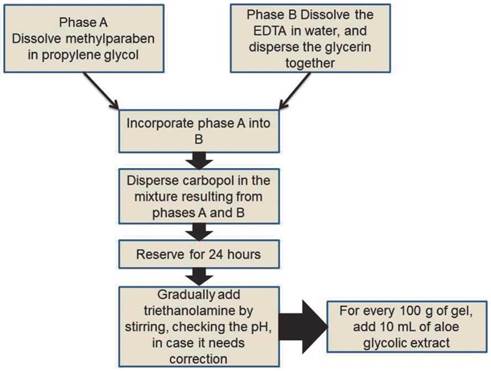
Table 1 shows the components present in the formulation of the Aloe vera gel, with their respective quantities specified for 100 g.
Table 1 Composition of Aloe vera gel.
| Components | Quantities |
|---|---|
| Carbopol 940 | 1 g |
| Glycerin | 5 g |
| EDTA | 0.10 g |
| Propylene glycol | 2.70 g |
| Methylparaben | 0.20 g |
| Triethanolamine | 1.15 g |
| Glycolic extract | 10 mL |
| Water | q.s.p 100 g |
Source: Adapted from Formulário Nacional da Farmacopéia Brasileira [11].
To produce the Aloe vera gel, the carbopol 940 gel shown in figure 2 was prepared.
pH determination
The pH was determined directly in a calibrated pH meter, as described in the Brazilian Pharmacopeia [12].
Determination of apparent density
The apparent density was determined in triplicate using 10 g of gel in heavy empty and filled beakers on an analytical balance and calculated from the formula [13]:
Determination of viscosity
The viscosity of the gel was evaluated by measuring the resistance to the rotation movement of metal axes when immersed in the liquid in a Brookfield rheometer [12]. Rotors 2, 3 and 4 were used, with a speed of 6 rpm and the calculation was performed using the following equation:
η = K.α
where, η is the absolute viscosity, K is the coefficient, α is the reading indicated by the pointer.
Determination of spreadability
To determine the spreadability, the technique proposed by Knorst [14] was used, which uses glass plates on a scale of graph paper to determine the surface that the sample covers by measuring perpendicular diameters, with subsequent calculation of the area obtained in mm2. 1 g of the sample was deposited in the central space of the plate (20 x 20 cm), after which a glass plate of known mass was placed on the sample. After three minutes, the diameters covered by the sample were read in a horizontal position, using the graph paper and then the spreadability was calculated. This procedure was repeated by successively adding 250 g, 500 g, and 750 g weights in intervals of three minutes from one weight to another, the procedure was performed in triplicate. The spreadability of the samples was determined according to the added weight, according to the equation below [15].
where, Ei: Sample spreadability for a given weight in square millimeter (mm2), d: Average diameter in millimeter (mm).
The spreadability factor (FE) was also calculated by:
where, Ei = maximum spreading area (mm2) and P = total added weight (g).
Centrifugation test
The centrifugation test produces stress in the sample, anticipating possible instabilities of the product, through a simulation in the increase in the force of gravity, and increasing the mobility of the particles [13]. 5 g of gel was weighed and placed in the centrifuge at three different rotations, respectively, 1000 rpm, 2500 rpm, 3000 rpm. For 15 minutes [16].
Organoleptic characteristics
The obtained gel was observed visually, in terms of appearance, color and odor [13].
RESULTS AND DISCUSSION
Organoleptic characteristics
Considering the suitability for the treatment of wounds by topical application, the gel obtained was evaluated for its physical-chemical properties. Such a semi-solid formulation is characterized as an aqueous vehicle with pleasant sensory and easy application on the skin. The gel containing glycolic extract of Aloe vera obtained, was homogeneous, translucent, shiny, odorless and without lumps as observed in figure 3.
The gel formulation is the choice, among topical semi-solid manipulation options, because in addition to ease of administration, it has high viscosity, good permanence, moisturizing effect on scaly skin, greater bio-adhesion and less potential for irritation [17].
Physical-chemical characteristics
The physical-chemical characteristics of the gel obtained are shown in table 2.
pH
The pH value (5.44) was similar to that found by Borella et al. [18], in which he highlights small pH variations using Carbopol 940 in papain gels. The gel showed values according to the cutaneous pH which is slightly acid varying from 4.6 to 5.8, this acidity is a way of protecting the skin against microorganisms [19, 20]. The pH is an important parameter with regard to acute wounds since they have an alkaline pH, which predisposes to less healing, while acidic conditions help in this process [21, 22].
Apparent density
The apparent density of the aloe gel was 0.1018 ± 0.0008 (g/mL) and represents the relationship between the mass and the volume occupied by the sample. In liquids or semi-solids this parameter may indicate the incorporation of air or the loss of volatile ingredients in the sample [13]. According to Pedrazzi et al. [23] the measure of the product density is dependent on the characteristics of the components present in its formulation, and also on the existence or not of incorporated air during the mixing process.
Viscosity
It was not possible to obtain an accurate viscosity value because it exceeded the maximum reading limit of the equipment used. Following the proposed formula and considering the maximum possible reading value (100), it can be said that the sample has viscosity above 10 000 cP. In addition, it is important to highlight that this finding is expected, since, according to Handbook of Pharmaceutical Excipients, carbomers such as Carbopol 940 have viscosity that can vary between 40 000 to 60 000 cP [24, 25].
The present study corroborates the research by De Aguiar [24] who tested gels based on Carbopol at different temperatures, including body temperature, in which it had a high viscosity, reinforcing that this group of gels has high consistency and good adherence. The evaluation of this component helps to define whether a product indicates the appropriate consistency or fluidity and can show whether the stability is adequate [13]. Thixotropy is a rheological characteristic referring to changes in viscosity with time of application [26].
Topically applied formulations should preferably be viscous when inert and become more fluid when administered to the skin [27]. The rheological characteristic referring to the change in viscosity with the application time is thixotropy [26], and according to Mateus et al. [22], an increase in the viscosity of the gel prolongs the release of the drug causing it to improve patient compliance with treatment as it will reduce the number of applications.
The type of polymer and its concentration influence the thixotropic characteristic of the gel. Base formulation with Carbopol presents a higher thixotropy, which is desirable together with its high viscosity, due to deformation in the application and soon after resuming its initial viscosity [28].
Spreadability
The expansion capacity of the semi-solid formulation against a given force (weight) for a time is defined as spreadability. Its determination is essential, as it is related to the ease of topical application of the product [29]. Spreadability is based on resistance to forced movement. The results correspond to the relationship between the spreading area, which will be caused by a force applied on the gel [30].
The graphical representation of the spreadability as a function of the applied mass (figure 4) revealed rheological behavior ranging from 1994.65 mm2, when subjected to the weight of the glass plate, to 4042.97 mm2 with the additional mass of 750 g.
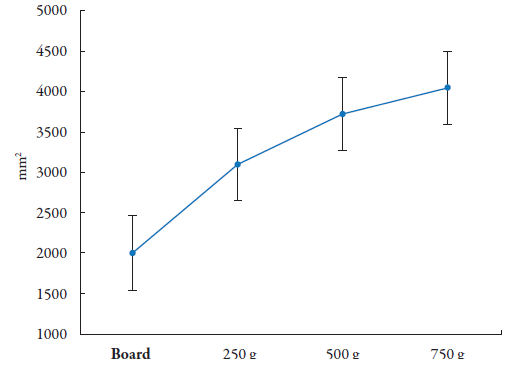
Source: Research Archives, 2019.
Figure 4 Representative graph of Aloe vera gel spreadability (n = 3).
The spreadability factor of the aloe gel obtained with 1% carbopol was 8.40 ± 3.6 mm2/g. Carbopol Ultrez hydrogels obtained by Paines et al. [31] containing clotrimazole and tea tree oil showed spreadability factors around 4.6 mm2/g, but it is not clear the percentage of the polymer in the formulation.
Cordeiro et al. [30], in a technological development study and evaluation of the stability of dermatological gel based on ginger essential oil, showed that gel based on Carbopol 940 maintains its spreadability without the product draining during administration, even in the face of changes of temperatures, since it was exposed to a preliminary and accelerated stability study showing good physical stability of the formulation which contains Carbopol.
The uniform application of the gel on the skin depends on the spreadability, a good gel will spread easily, the spreadability will help the patient's adherence to the treatment as well as an easier application [32]. A good criterion for the gel to meet the qualities recommended by Organs regulatory bodies is the propagation capacity, which would be the extension of the area where the gel spreads easily at the time of use [33].
Centrifugation test
The centrifugation test represents a preliminary stability test, since, with increased gravity, it is demonstrated in advance if the product will show any type of instability [34]. After centrifugation in all conditions established for the test, the sample remained stable and without change in coalescence, phase changes, color, or odor, showing itself stable as seen in figure 5.
Cordeiro et al. [30], and Coelho et al. [34] obtained similar results in preliminary stability in dermatological gel from ginger essential oil and gel containing guava leaf extract, respectively. The stability research contributes to: Guide the development of the formulation and packaging material, serve as alternatives for improving the formulations, help to also determine the expiration date and control of organoleptic, physical-chemical and microbiological stability, producing information on the quality of the product. On the other hand, rheology represents the study of the flow and deformation properties of matter under the action of forces, whose behavior helps in the early detection of signs of instability in formulations, assuming paramount importance for the evaluation of semi-solid formulations [35, 36].
CONCLUSIONS
The gel formulated from glycolic extract of Aloe vera showed satisfactory organoleptic characteristics, with a homogeneous and shiny appearance, with no apparent odor and translucent color, without altering these characteristics; slightly acid pH, compatible with the skin, which favors wound healing; adequate rheological aspects with good viscosity and spreadability; no changes related to stability, under the conditions evaluated. The development of gels must be carried out consistently and taking into account the therapeutic potential of the present formulation for the treatment of wounds, it is suggested complementary studies regarding the microbiological quality, guaranteeing the safety of a product that presented a pleasant appearance and rheological characteristics, and promising stability.