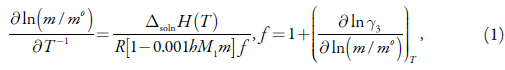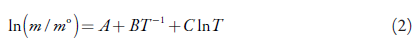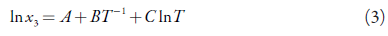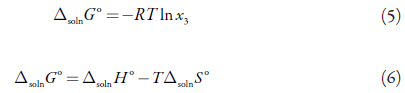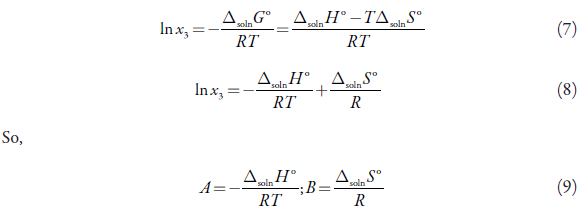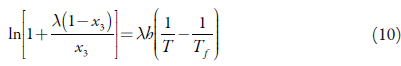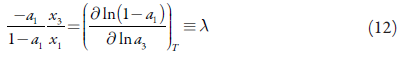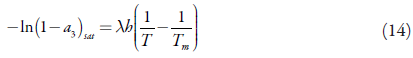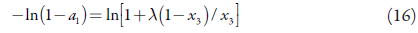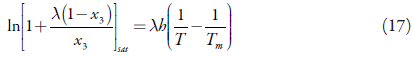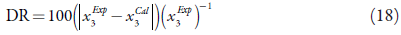INTRODUCTION
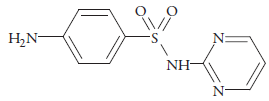
Sulfadiazine (iV-2-pyrimidinylsulfanilamide) (figure 1) is a member of the sulfonamide family [1]. As one of the earliest antibacterial drugs used in the prevention and treatment of bacterial infections, sulfonamides are widely used in the medical and livestock industries due to their broad antibacterial spectrum, stability and low-cost [2-4], however, sulfadiazine solubility in ambient water is very low and it is considered as practically insoluble [5-8]. Although the solid dosage form of sulfadiazine has been widely used in therapeutics, the development of liquid dosage forms is important to administer the drug to special groups of patients. To increase the solubility of sulfadiazine some cosolvents have been added to water [9, 10]. The analysis of sulfadiazine in the pharmaceutical industry typically involves high-performance liquid chromatograph that consumes a large quantity of hazardous organic solvents and its waste needs to be properly disposed of [11, 12].
However, solubility measurement and optimization of the solvent composition for dissolving a desired amount of a drug in a given volume of the solution in most of the cases is a laborious and time consuming procedure which may cause some limitations in discovering and development of new drugs [13, 14]. Mathematical model, as an alternative method, could be used to estimate drug's solubility especially when the solid-liquid equilibrium measurements become impractical. Various numerical models proposed for the estimation of the solubility of drug and/or drug like compounds in the cosolvency systems which in order of appearance include van't Hoff equation [15], Yalkowsky model [5, 16, 17], the mixture response surface model, the double log-log model, the modified Wilson model[18], the Jouyban-Acree model [19-21], the Jouyban-Acree-van't Hoff model, and modified Jouyban-Acree-van't Hoff model. Van't Hoff equation predicts solubility at different temperatures in each solvent composition [22, 23], whereas Yalkowsky model, the modified Wilson model [18], the double log-log model and mixture response surface model predict solubility at different solvent mixtures. Whereas, the Jouyban-Acree [24] andJouyban-Acree-van't Hoff models [25] predict the solubility in various solvent composition at different temperatures.
The objective of the present work is to evaluate the application of the van't Hoff, Buchowski-Ksiazaczak λh, and modified Apelblat models to the solubility of sulfadiazine in three cosolvent systems.
THEORETICAL
Modified Apelblat equation
From the Williamson equation, the solubility of non-electrolytes is expressed as [26]:
where h denotes a number of water molecules in the hydrate, Δsoln H(T) is the molar enthalpy of solution, y3 is the activity coefficient of the solute, M1 is the molar mass of solvent, R is the gas constant and m° = 1 mol.kg-1.
If it is assumed that the enthalpy of solution depends linearly on the temperature, the integral form of equation (1) is [27]:
So, expressing the solubility in mole fraction, the modified Apelblat equation, which is a semi-empirical model derived from solid-liquid equilibrium is [28]:
x 1 refers to measured mole fraction solubility of drug in selected solvent at absolute temperature T; A and B reflect the variation in the solution activity coefficient; C represents the influence of temperature on enthalpy of fusion [29, 30]; The above three parameters and experimental prediction values can be obtained by the nonlinear regression method in Python.
Van't Hoff equation
The van't Hoff equation is also a semi-empirical equation, which reveals the relationship between the mole fraction solubility and the temperature in an ideal solution by taking the solvent effect into account. The formula is shown as equation (4).
A and B are parameters, which can be related to thermodynamic parameters such as dissolution enthalpy and dissolution entropy [22]. he above can be shown from the two mathematical expressions for the Gibbs energy [31-34]:
Solving for Δsoln G° from equation (5), subsequently replacing Δsoln G° from equation (6), we obtain,
Buchowski-Ksiazaczak λh model
In 1980, based on the generalized relationship, the Buchowski-Ksiazaczak λh equation was first proposed, which contains two parameters and is widely used to correlate solubility data. The Buchowski-Ksiazaczak λh equation is shown in equation (10).
where λ and h are the two parameters of the λh model, and T f represents the melting point of drug [35-37]. he model can be tested starting from the following identity [38]:
Integrating the identity -equation (11)- into the equation of into the Gibbs-Duhem equation, we obtain,
In this way λ is related to activity and therefore to the concept of ideality.
Buchowski showed that when graphing ln (1 - α) vs 1/ T, a linear function was obtained whose slope is λh
Integration of equation (13) from T m to T gives equation (14).
where T m is the melting temperature.
For the solubility curve, equation (14) is rearranged as:
Hence,
So, equations (14) and (16) give the solubility curve:
Equation (17) is equal to equation (10).
RESULTS AND DISCUSSION
The solubilities of sulfadiazine in mixtures of (methanol + water), (ethanol + water) and (1-propanol + water) were provided by Delgado and Martínez [6-8].
The experimental solubility data were correlated with the van't Hoff equation, Apelblat equation and Buchowski-Ksiazaczak λh equation, using the software with the Python version 3.8 using pandas and NumPy library, the parameters of the models were calculated with SciPy library and plots were made with Plotly library.
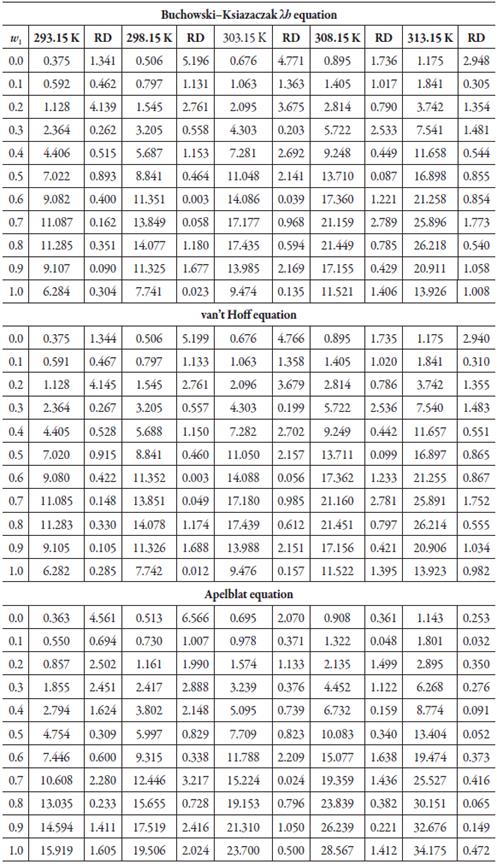
Table 1 shows the parameters of the equations of λh, van't Hoff and Apelblat, for the solubility of SD in (methanol + water) co-solvent mixtures. Although Delgado and Martínez implements the van't Hoff equation, the purpose is to calculate the thermodynamic functions as shown in other references [39-42], and not, to use the equation to calculate the solubility at different temperatures regarding the experimental ones [43, 44]. The melting temperature data, necessary for the development of the λh model, was taken from the literature (259.5 °C) [6].
Table 1 The λh, van't Hoff and Apelblat equations parameters for sulfadiazine in (methanol + water) cosolvent mixtures.
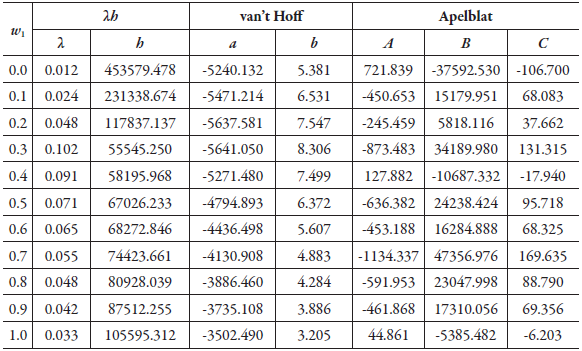
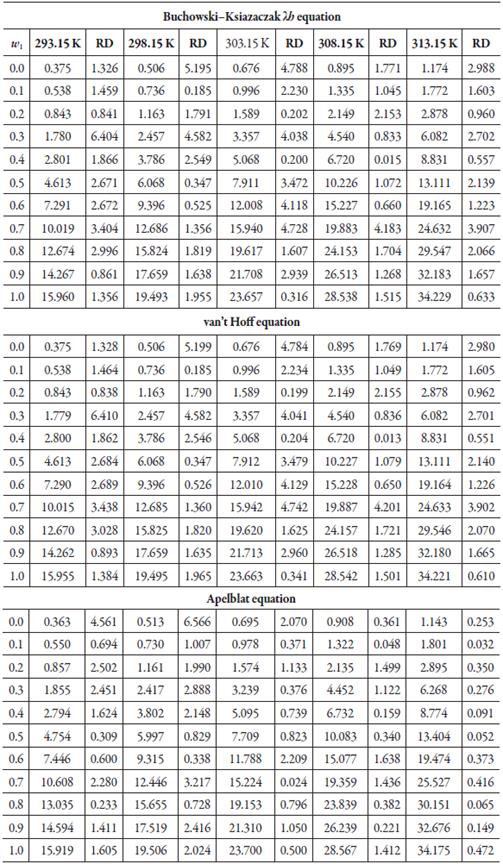
Table 2 shows the calculated solubility using each of the exposed models, and the relative deviation, calculated using equation (18) [45-48].
Table 2 Calculated solubility of sulfadiazine in (methanol + water) mixtures expressed in mole fraction (104 x3), from equations of λh, van't Hoff and Apelblat.
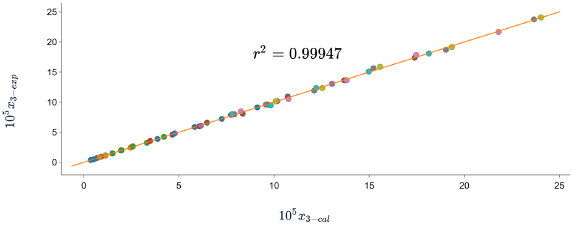
In all cases, the DR of the data calculated with respect to the experimental data is less than 7%. which indicates that the three models predict the solubility of SD in (methanol + water) cosolvent mixtures with good precision. When graphing the experimental data vs. the calculated data (figures 2, 3 y 4), linear holdings are obtained with correlation coefficients equal to 0.99, corroborating that these models show good agreement with the experimental solubility data.
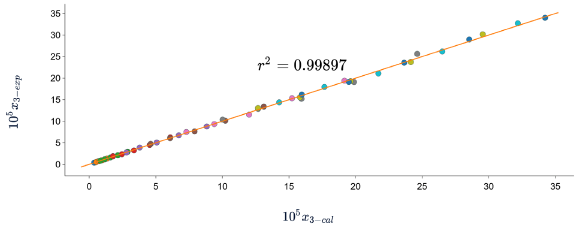
Figure 2 Experimental solubility vs calculated solubility by λh model of sulfadiazine in (methanol + water) mixtures.
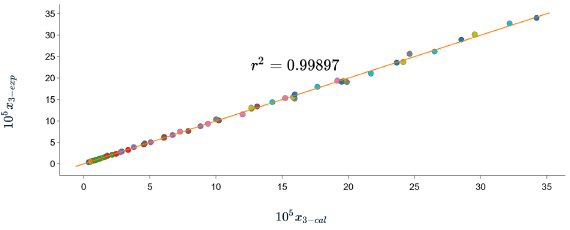
Figure 3 Experimental solubility vs calculated solubility by van’t Hoff model of sulfadiazine in (methanol + water) mixtures.
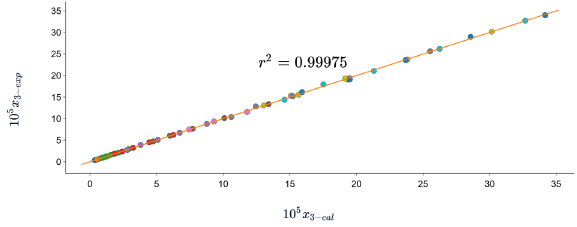
Figure 4 Experimental solubility vs calculated solubility by Apelblat model of sulfadiazine in (methanol + water) mixtures.
Table 3 shows the parameters of the equations of λh, van't Hoff and Apelblat, for the solubility of SD in (ethanol + water) cosolvent mixtures and table 4 shows the calculated solubility using each of the exposed models, and the relative deviation. As in the (methanol + water) cosolvent mixtures, the three models predict the solubility data of the SD, with good precision, in this case, the DR are less than 5%.
Table 3 The λh, van't Hoff and Apelblat equations parameters for sulfadiazine in (ethanol + water) cosolvent mixtures.
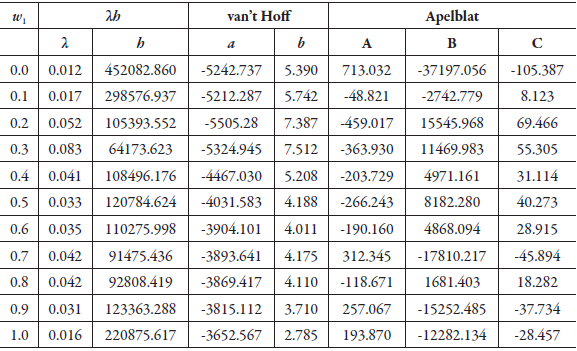
Table 4 Calculated solubility of sulfadiazine in (ethanol + water) mixtures expressed in mole fraction (104x3), from equations of λh, van't Hoff and Apelblat.
Figures 5, 6 and 7 show the correlation between the experimental solubility data of SD in (ethanol + water) cosolvent mixtures and those calculated with the three models, in all cases the correlation coefficients are equal to or greater than 0.99.
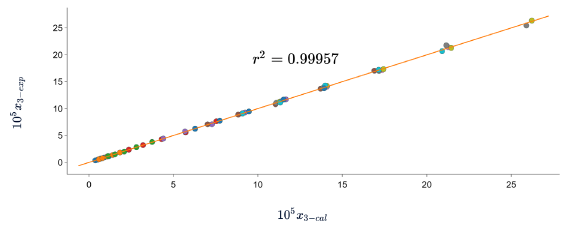
Figure 5 Experimental solubility vs calculated solubility by λh model of sulfadiazine in (ethanol + water) mixtures.
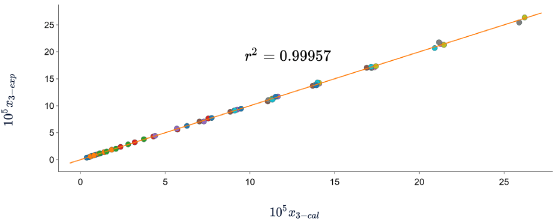
Figure 6 Experimental solubility vs calculated solubility by van't Hoff model of sulfadiazine in (ethanol + water) mixtures.
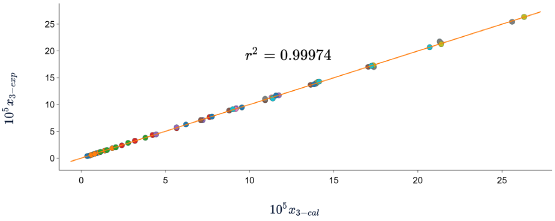
Figure 7 Experimental solubility vs calculated solubility by Apelblat model of sulfadiazine in (ethanol + water) mixtures.
Table 5 shows the parameters of the equations of λh, van't Hoff and Apelblat, for the solubility of SD in (1-propanol + water) cosolvent mixtures and table 6 shows the calculated solubility using each of the exposed models, and the relative deviation.
Table 5 The regression parameters of the Apelblat, van't Hoff, and λh model for sulfadiazine in (1-propanol + water) mixtures.
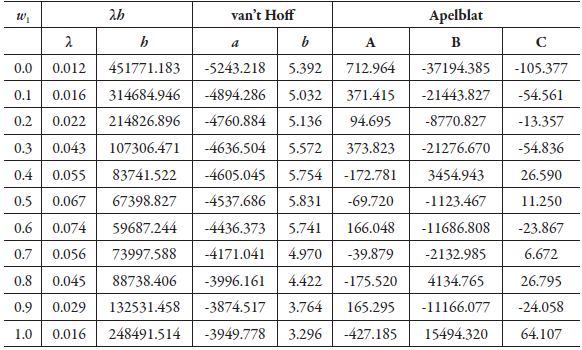
Table 6 Calculated solubility of sulfadiazine in (1-propanol + water) mixtures expressed in mole fraction (104x3), from equations of λh, van't Hoff and Apelblat.
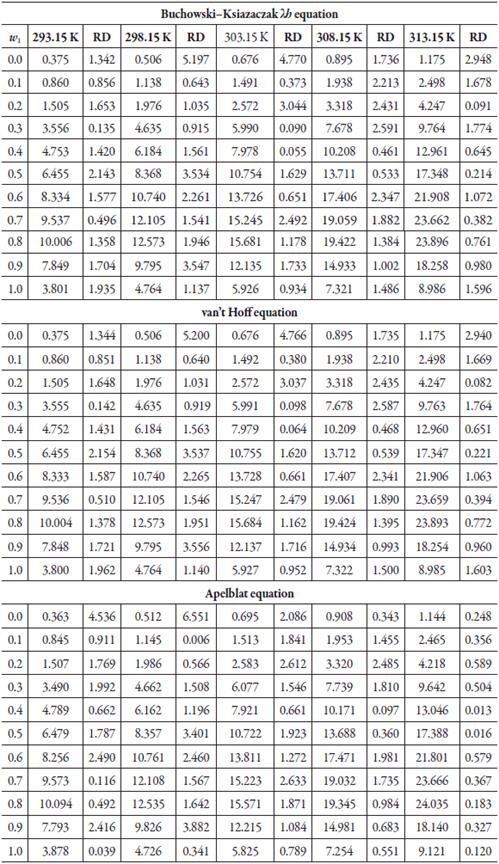
According to the results shown in table 6 and figures 8, 9 and 10, in which the experimental and calculated solubility are compared. the three solubility models, including Apelblat model, Buchowski-Ksiazaczak λh equation and van't Hoff model, were applied to fit with experimental data obtaining a good predictability of the experimental data, since the DR values do not exceed 5%.
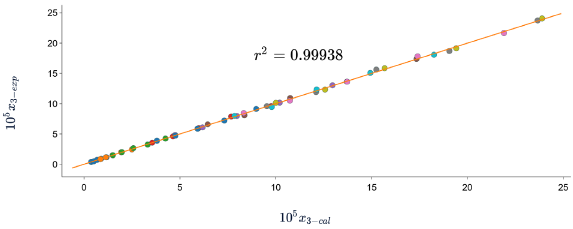
Figure 8 Experimental solubility vs calculated solubility by λh model of sulfadiazine in (1-propanol + water mixtures) mixtures.
Figure 9 Experimental solubility vs calculated solubility by van't Hoff model of sulfadiazine in (1-propanol+ water) mixtures.
In general terms, from the results shown in tables 2, 4, 6 and figures 2-10, it can be observed that the Apelblat model, Buchowski-Ksiazaczak λh equation and van't Hoff model gave almost the same goodness of fit to the experimental data. Error percentages of the predicted solubility values obtained from calculations using both models were below 5%, except for one point of SD solubility in (methanol + water) mixtures (w 3 at 293.15 K). The goodness of fit of these equations can also be seen from the r2 values listed in figures 2-10, which are almost unity. Buchowski et al. [38] stated that in an ideal solution, the value of 1 equals to 1. The values of λ the obtained equation parameter (λ) in tables 2, 4 and 6 showed that the SD in (methanol + water), (ethanol + water) and (1-propanol + water) cosolvent mixtures, understandably were nonideal. This agrees with Delgado and Martínez [6-8], who reported that solution of SD in some cosolvent mixtures are highly non-ideal.