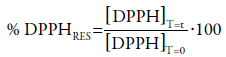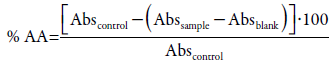INTRODUCTION
Plants sustain life on Earth, performing extraordinary functions because they are the essence of the food chain. In addition, they supply oxygen to all living things, and possess medicinal properties. Indeed, Brazil stands out for the significant market share of its herbal products and for its research efforts aimed at the development of new drugs [1].
Little research has so far focused on the use of secondary metabolites such as phenols, tannins, and flavonoids, which have a potential for antioxidant activity, to substitute some synthetic food additives, and on identifying other possible health benefits of antioxidants. Therefore, quantitative, and qualitative research into these metabolites has become increasingly relevant. The identification of natural bioactive compounds is of utmost importance in the search for new drugs. Phytochemical compounds are found in various combinations in different parts of the plant (leaves, roots, shoots, bark), in different stages of growth (germination, maturation), and under different environmental pressures [2].
Azadirachta indica A. Juss (neem) is a plant of Asian origin, of the family Meliaceae, native to Myanmar and the arid regions of India [3]. The species has been used for centuries in the East as a medicinal plant (in the treatment of inflammation, viral infections, hypertension, and fever), as a shade tree, an insect repellent, as construction material, fuel, lubricant, fertilizer, and pesticide [4]. The leaf extract cab act as a growth promoter [5]. Other authors have also investigated and identiied other functions related to improved performance, hematological parameters [6] and immune response in animals [7, 8]. Compounds extracted from the plant are selective and non-mutagenic, biodegrading rapidly, with low toxicity to non-target organisms and with minimal disturbance to ecosystems [9].
A. indica seeds and leaves are widely used to control pests [10, 11]. This behavior is largely due to the presence of toxic triterpenes, with emphasis on azadirachtin and some of its derivatives [12, 13]. Much higher concentrations of azadirachtin have been found in the seeds than in other parts of this plant [14]. In addition to azadirachtin and its derivatives, other substances with interesting biological applications against insects have been isolated, such as vilasinin [15], salanin and meliantrol [16].
This study focused on the phytochemical identification of different classes of secondary metabolites present in the hydroethanolic extract of A. indica (neem) leaves, as well as an investigation into their antioxidant and toxic potential.
MATERIAL AND METHODS
Collection and processing the plant material
The leaves of Azadirachta indica A. Juss (neem) used in this study were collected in September 2016 in the Zeca Ribeiro neighborhood of the municipality of Barra do Garças, state of Mato Grosso, Brazil, at the geographical coordinates of 15°54'03.5"S 52°16'39.1"W. Using a knife and pruning shears, about 5 kg of the material were collected for the chemical study and analysis. The plant was taxonomically identified at the Herbarium of the Federal University of Mato Grosso (UFMT/CUA) - Unit I, Pontal do Araguaia (MT), registered under number 10 216. Initially, the leaves were dried without exposure to the sun, at room temperature for 7 days. The branches of the leaves were then removed and only the leaves were ground into a powder in a blender.
Preparation of the crude extract of Azadirachta indica A. Juss (neem) leaves
The powdered plant material (2558.0 g) was extracted for about 45 days with approximately 12 L of a solution of ethanol: distilled water (8:2) at room temperature, protected from light, with occasional shaking. After this period, the extract was filtered to separate the solid material from the hydroethanolic extract. The solvent was recovered by rotary vacuum evaporation (Quimis) at 60 °C. The crude leaf extract was then oven-dried at 50-60 °C for about 7 days until it reached a constant weight, whereupon its yield was calculated. After this process, the crude extract was then stored at 7-10 °C to prevent its degradation.
Phytochemical analysis
Phytochemical tests were performed in seven test tubes, as described by Matos [17], with minor adaptations, based on chemical reactions with specific reagents, which will be described below, based on color changes. Initially, 0.5 g of the crude dry extract from A. indica leaves were suspended in 50 mL of ethanol: distilled water (8:2), and 3 mL of this solution was poured into each test tube for analysis. The secondary metabolites described below were then examined.
Phenols and tannins
Three drops of 5% ferric chloride (FeCl3) ethanolic solution were poured into tube 1, which was then shaken vigorously, causing a change in color or the formation of a precipitate. The appearance of a color varying between blue and red is indicative of the presence of phenols (negative blank test), while the formation of dark blue precipitate indicates the presence of pyrogallic tannins (hydrolyzable tannins) and green indicates that of condensed or catechin tannins.
Anthocyanins, anthocyanidins, flavonoids, chalcones and aurones
In this experiment, tubes 2, 3 and 4 were used. Tube 2 was acidified to pH 3.0 by adding 0.1 mol.L-1 of aqueous hydrochloric acid solution (HCl), while tubes 3 and 4 were alkalinized to pH 8.5 and 11, respectively, by adding 0.1 mol.L-1 of an aqueous solution of sodium hydroxide (NaOH). The color change in each tube indicated the presence of several chemical constituents, as described in table 1.
Table 1 Color change indicating the presence of different constituents, according to pH level.
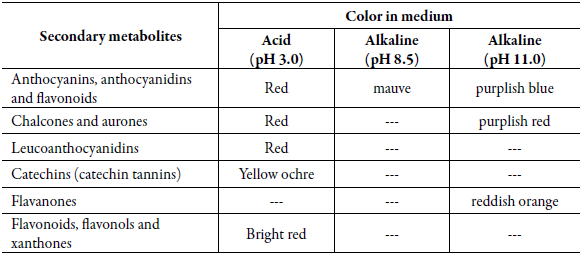
Source: Adapted from Biswas et al. [17].
Leucoanthocyanidins, catechins and flavanones
The content of tube 5 was acidified to a pH level of 1.0-3.0 by adding an aqueous solution of 0.1 mol.L-1 of HCl. Tube 6 was alkalinized to pH 11.0 by adding an aqueous solution of 0.1 mol.L-1 of NaOH. The tubes were then heated carefully over a Bunsen burner for 2 to 3 min. A comparison of the color of the contents of these tubes with the corresponding tubes used in the previous test revealed changes. The appearance or intensiication in color indicated the presence of the constituents listed in table 1.
Flavonoids, flavonols and xanthones
A few milligrams of magnesium granules and 0.5 mL of concentrated HCl were added to tube 7. At the end of the reaction, which was indicated by the end of effervescence, a color change was observed when compared with tube 5 used in the previous test (both acidified). The appearance or intensification of the red color indicates the presence of flavonoids, flavanonols and/or xanthones in their free forms or their heterosides.
Quantitative antioxidant activity determined by UV-Visible spectroscopy (UV-Vis)
Literature procedures were followed [18, 19], with a few adaptations.
Preparation of the DPPH solution for the test
A stock solution of 50 mL of the radical 2,2-diphenyl-1-picrylhydrazyl (DPPH-Sigma-Aldrich), weighing 0.0125 g, was prepared in an analytical balance (Metler) and diluted in methanol to the desired volume, resulting in a final solution of 250 μg.mL-1. Seven dilutions were the prepared from the stock solution, in the following concentrations: 1, 5, 10, 15, 20, 25 and 30 μg.mL-1. These solutions were protected from light by means of aluminum foil and stored in a refrigerator until used.
The standard curve was drawn from the absorbance value at 515 nm, obtained in an SP 2000 UV-Vis spectrophotometer. This standard curve was obtained using approximately 3.0 mL of the solutions, which were transferred to glass cuvettes with an optical path length of 1.0 cm, where the reading was performed, using methanol (Vetec) as blank. Absorbance measurements were taken in triplicate, with a 1-min interval between each reading.
Dilutions of the extract and the positive control
A quantity of 0.0500 g of the crude extract was carefully weighed on an analytical balance (Metler) and this amount was dissolved in 25 mL with methanol (Vetec), resulting in a concentration of 2.000 μg.mL-1. Solutions with concentrations of 25, 50, 100, 150, 200 and 250 μg.mL-1 were prepared by dilution from this stock solution and were used to perform the antioxidant test.
Rutin (Sigma-Aldrich) was used for the positive control, employing the same dilution procedure as that of the extracts described above. The resulting solutions were kept away from light and stored in a refrigerator until the moment they were used for the tests.
Antioxidant testing procedure
The solutions used in this test were prepared using 0.3 ml of each concentration of the extracts and the control, to which 2.7 mL of the DPPH solution at 40 μg.mL-1 were added in test tubes protected from light. The absorbance readings were taken after 1, 5, 10, 20, 30, 40, 50 and 60 min. The blanks used here were 2.7 mL of methanol and 0.3 mL of each concentration of the extracts. For the positive control blank, 2.7 mL of methanol and 0.3 mL of each concentration of the control were also used.
Percentage of residual DPPH
The percentage of residual DPPH (% DPPHRES) was determined based on the equation of the calibration curve and the absorbance values over a period of 30 min for each concentration:
where [DPPH]T=t corresponds to the concentration of DPPH after the reaction with the extracts and positive control, while [DPPH]T=0 is the initial concentration of DPPH, i.e., 40 μg.mL-1.
Percentage of antioxidant activity
The absorbance values of all the tested concentrations were converted into percentage of antioxidant activity (% AA), determined by the following equation:
where Abscontrol is the initial absorbance of the DPPH solution and Abssample is the absorbance of the reaction mixture (DPPH + sample).
Evaluation of the potential toxicity of Azadirachta indica (neem) leaves
The potential toxicity of the crude hydroethanolic extract obtained from leaves of A. indica against larvae of Artemia salina Leach was evaluated according to the procedures proposed by Meyer et al. [20], with some modifications, and to descriptions in the literature [21-23].
A. salina larvae were obtained from eggs supplied by the company Artemia Salina do RN. These eggs were placed for 24 h in an aqueous solution of 0.037 g.mL-1 (m/v) of sea salt (Mossoró) to hatch. The pH level of the solution was adjusted to 7.0 by adding 0.1 mol.L-1 aqueous NaOH solution), under constant aeration and light from a 40W lamp (around 27-28 °C).
A standard solution with a concentration of 5 mg.mL-1 was then prepared by diluting 50 mg of the crude extract in methanol. From this standard solution, 15, 50, 100, 500, 600 and 700 μL were transferred to test tubes, which were heated in an oven at 50 °C until the solvent was completely evaporated. This procedure was performed in triplicate.
After total evaporation of the solvent, 3 mL of saline solution were added to each tube, followed by 10 A. salina larvae, and the volume of the test tube was completed by adding up to 5 mL saline solution. The final concentrations in the test tubes were 15, 50, 100, 500, 600 and 700 μg.mL-1.
The control tube was prepared containing only 5 mL of saline and 10 A. salina larvae. After 24 h of exposure, the number of surviving larvae was counted with the aid of a magnifying glass and a glass rod. Larvae that remained immobile for more than 10 seconds after mildly shaking the tubes were considered dead. The average lethal concentration (LC50) of each investigated ester and starting compounds was then calculated based on the concentrations under study, using StatPlus 2008 software [24].
RESULTS AND DISCUSSION
Characteristics of the powdered material, crude extract, and yield
The dried neem leaves were ground into a green powder in order to prepare the crude hydroethanolic extract. The dry crude extract was dark green, with a pasty consistency and a strong odor characteristic of plant material. A total amount of 197.0 g of crude extract was obtained (7.7% yield).
Phytochemical analysis
Table 2 shows the results of the qualitative analysis of the metabolites investigated in this work. The change in color indicative of the presence of the classes of these substances, according to the literature [17] is described in table 1.
Table 2 Secondary metabolites identified in the crude hydroethanolic extract obtained from the leaves of Azadirachta indica (neem).
| Class of metabolites investigated | Result |
|---|---|
| Phenols | ( + ) |
| Tannins (condensed or catechin) | ( + ) |
| Tannins (pyrogallic or hydrolyzable) | ( - ) |
| Anthocyanins, Anthocyanidins and Flavonoids | ( - ) |
| Chalcones and Aurones | ( - ) |
| Leucoanthocyanidins | ( + ) |
| Catechins (catechin tannins) | ( + ) |
| Flavanones | ( - ) |
| Flavononols, Flavonols and Xanthones | ( + ) |
Note: ( + ) = positive; ( - ) = negative.
The chemical method used in the analysis of phenols (tube 1) was based on the reaction of the phenolic groups with the ferric chloride reagent, forming iron complexes and producing blue pigments [25]. Thus, it was reactive to the presence of phenolic compounds in the sample, presenting a darker color than that of the blank, which consisted only of ferric chloride solution.
Tube 1 was also used to identify tannins. The addition of ferric chloride drops to the aliquot of the extract solution (tube 1) would immediately produce a bluish precipitate in the presence of hydrolyzable tannins (pyrogallic), or a greenish precipitate in the presence of condensed tannins (catechin), thus rendering the reaction specific in determining the two classes of tannins, as shown in table 1. These compounds are easily oxidized by specific plant enzymes and by metals such as the ferric chloride used here, causing the solutions to darken [26]. The reaction produced a dark green precipitate, which, according to the literature, confirms the presence of condensed or catechin tannins, and hence, the absence of pyrogallic or hydrolyzable tannins.
No compounds were detected in the tests to identify anthocyanins, anthocyanidins and flavonoids in tubes 2, 3 and 4, in view of the absence of red in the acidic medium (pH 3.0), of mauve in the alkaline medium (pH 8.5) and of purplish blue in the alkaline medium (pH 11.0). The same applies to the chromatic tests for chalcones and aurones, which showed no changes in the colors of tubes 2, 3 and 4.
Positive results were obtained for the presence of leucoanthocyanidins and catechins (catechin tannins), indicated by darkening of the color in tube 5 and the absence of color change in tube 6. The presence of catechin tannins had already been revealed in the test with ferric chloride (tube 1). As for flavanones, the result was negative (tube 6), as indicated by the absence of a reddish orange hue, and the color remained purplish red (tube 4, pH 11.0, without heating).
Tube 7 showed a positive result for flavonoids, flavonols and xanthones. This reaction is caused by the reduction of yellow flavonoid derivatives, which take on a reddish color in the presence of magnesium in a strongly acidic medium, and a bluish color in the presence of anthocyanins, as previously reported [27]. The reaction was positive since the color tube 7 intensified.
The results in table 2 indicated the presence of secondary metabolites commonly related to antioxidant properties, mainly phenols, tannins and catechins. Phytochemical studies with alcoholic extracts of A. indica in semi-arid regions of Nigeria indicated moderate amounts of phenols, tannins, and flavonoids [28].
The presence or absence of certain classes of metabolites present in the leaves of A. indica may vary according to geographical position and climatic aspects [29]. This statement is shared by Ghimeray et al. [30], in their studies of quantification ofpheno-lic derivatives of A. indica leaves from Nepal.
The plant A. indica (neem) is originally from Asia and having been adapted in several parts of the world, including Brazil. Many studies of the plant are reported in the literature related to different biological activities, among them the antioxidant activity. More than 140 substances have already been isolated from different parts of the plant (bark, flowers, leaves, fruits, roots, and seeds), many of these biologically active substances [31].
Quantitative antioxidant activity determined by UV-Visible spectroscopy (UV-VIS)
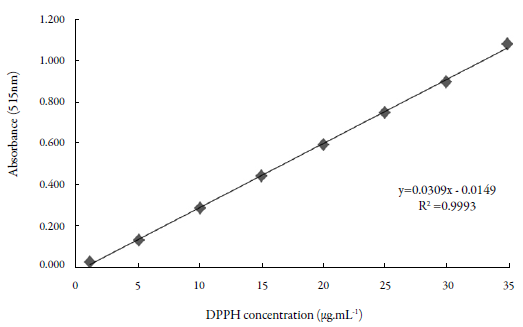
The calibration curve of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical in the test was A = 0.0309C + 0.0149, where C corresponds to the concentration of DPPH in the medium, A is the absorbance measured at a wavelength of 515 nm, and the correlation coefficient R = 0.9999, which was determined using StatPlus 2008 software (figure 1).
From the calibration curve and the absorbance values over 30 min at each tested concentration of the sample and the positive control, the residual DPPH values (% DPPHrRES) (figure 2) were determined using the equation (% DPPHRES = [DPPH]T=t /[DPPH]t=0 x 100), where [DPPH]T=t = C, as mentioned in the materials and method section.
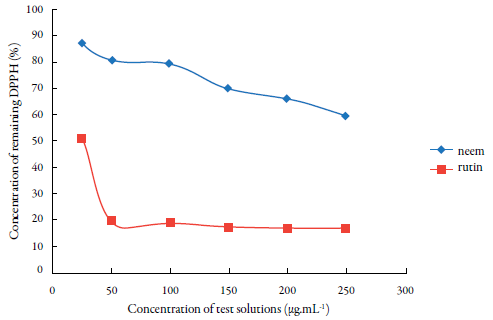
Figure 2 Percentage of residual DPPH after the reaction with different concentrations of hydroal-coholic extract from leaves of neem (A. indica) and rutin.
The results illustrated figure 2 confirm the moderate antioxidant activity of neem, indicated by a decrease in the amount of residual DPPH. However, when the highest tested concentration was used (250 μg.mL-1), 59.06% of the free radical was still present in the radical form.
Figure 3 compares the antioxidant potential of neem leaves and rutin (control). This comparison reveals a difference between the compounds, with the crude hydroethanolic extract of neem leaves showing moderate antioxidant activity, albeit still able to properly sequester the DPPH present in the reaction medium.
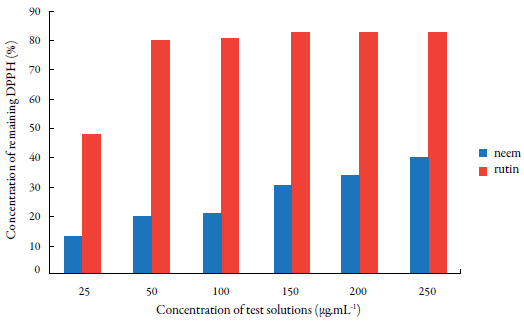
Figure 3 Percentage of antioxidant activity with different concentrations of hydroalcoholic extract from leaves of neem (A. indica).
The absorbance values at all the tested concentrations, within a 30-min period, were also converted into percentage of antioxidant activity (% AA), determined by the equation (% AA = {[Abscontrol - (Abssample - Absblank)] x 100}/ Absurd), where Abscontrol is the initial absorbance of the methanol solution of DPPH and Abssample is the absorbance of the reaction mixture (DPPH + sample). The % AA value found in the sample was 40.3%, which can be considered a moderate degree antioxidant activity.
Analysis of the antioxidant activity of A. indica (neem) in Nepal [30] indicated % AA values of 33% for crude methanolic extract obtained from the leaves, a result in line with that obtained in this work, while 82.5% for the bark extract. Antioxidant properties of A. indica leaves present in India and England have also been reported [32, 33].
Phenolic compounds are secondary metabolites responsible for the antioxidant activity exhibited by several sources of natural products. In China, this relationship was well evidenced in a study with 112 traditional plants, among them with leaves of A. indica (neem) [34].
This moderate antioxidant activity was confirmed by the phytochemical analysis. We also found that the absence of a part of the phenolic compounds, particularly the flavonoids present in neem leaves, indicates a decrease in the potential antioxidant activity. Thus, the antioxidant capacity obtained was in large part due to the presence of phenolic groups in the chemical structures of the secondary metabolites, mainly in phenols and tannins, which are markedly present in the crude hydroethanolic extract of A. indica leaves.
Evaluation of potential toxic effects on Artemia salina Leach larvae
The potential toxicity of the crude hydroethanolic extract of Azadirachta indica leaves was tested, using as parameter the lethal concentration sufficient to kill 50% of Artemia salina larvae, LC50, after 24 h of exposure to concentrations of 15, 50, 100, 500, 600 and 700 μg.mL-1 of saline solution of the crude extract of A. indica leaves. Figure 4 depicts the dose-response curve of the test performed.
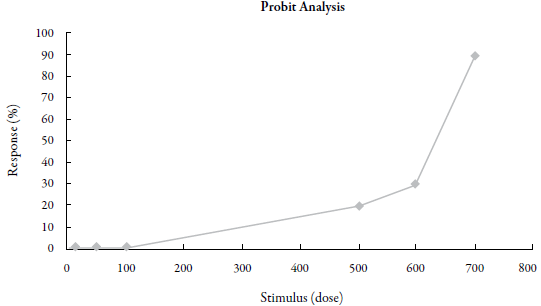
Figure 4 Analysis of the toxicity of crude hydroethanolic extract of A. indica (neem) leaves against Artemia salina larvae. The x-axis represents the dose of the extract in jig.mL1 and the y-axis represents the effect in percentage of deaths.
Studies have been published describing the standard use of Artemia salina larvae to determine the toxic effects of pure substances [35, 36], of plant extracts [37], and even of nanoparticulate systems [38]. Our study used as reference the standard proposed by Nguta et al. [37], because it clearly involves studies on the toxic potential of a plant extract against A. salina larvae.
According to Nguta et al. [37], both organic and aqueous plant extracts whose LC50 is lower than 100 μg.mL-1 are highly toxic, while LC50 between 100 and 500 μg.mL-1 is moderately toxic, LC50 between 500 and 1000 μg.mL-1 corresponds to low toxicity, and LC50 above 1000 μg.mL-1 is nontoxic. The test to determine potential toxicity of the raw extract of A. indica (neem) leaves showed an LC50 value of 596.7 μg.mL-1, with a confidence interval (95% CI) of 549.6 to 647.9 fxg.mL-1. Thus, a comparison of our result with this reference indicates that the crude extract under study exhibits low toxicity.
Among the various parts of the plant A. indica (neem), results of high toxic potential have been found in seed oil [9, 14, 39]. Studies relate this high toxicity to the presence of some substances, mainly azadirachtin, vilasinin, salanin and meliantrol [12, 13, 15, 16], with emphasis on azadiractin. This substance was detected in high concentrations in A. indica seed oil in India [40, 41]. Bioinsecticides produced from neem seed oil are already commercially available, in line with some of the principles of Green Chemistry.
In contrast to seed oil, the presence of azadirachtin in other parts of the plant (leaves, branches, and bark) is not common [9, 14]. The amount of the azadirachtin present in the leaves of A. indica in southern India was about 33 times less than in the oil of the seeds, that is, practically zero [40]. This may justify the low toxicity value generally found in the extracts obtained from the leaves of neem [42], as occurred in this work.
According to literature [20], lethal concentrations below 1000.0 μg.mL-1 indicate the presence of potentially active substances. Therefore, the concentration of 596.7 μg.mL-1 indicates that crude hydroethanolic extract of Azadirachta indica (neem) leaves contains substances with potential medicinal properties.
CONCLUSIONS
This phytochemical study of the crude extract of A. indica leaves revealed the significant presence of several secondary metabolites such as phenols, tannins, leucoantho-cyanidins, flavonoids, flavonols and xanthones. This plant extract showed a moderate antioxidant potential against the DPPH radical, with 40.3% antioxidant activity (% AA) at the concentration of 250.0 μg.mL-1. This moderate activity may be related to the presence mainly of known metabolites with antioxidant potential, such as phenols and tannins, as well as the absence of flavonoids. The LC50 of the crude leaf extract (596.7 μg.mL-1) confirms its low toxicity, indicating its possible safe and beneficial use in popular home remedies. It also indicates that the crude extract may contain secondary metabolites with interesting pharmacological properties. This study should contribute to the development of future research projects focusing on other biological activities of Azadirachta indica A. Juss (neem).