INTRODUCTION
Cancer is a complex disease that involves the interruption of proliferation control, resistance to cell death, invasion, and metastasis [1]. Among the main resources used in cancer treatment are surgical removal, hormone therapy, radiotherapy and chemotherapy. Such resources are often used in conjunction [2].
Falzone et al. [3] reported the evolutionary importance of chemotherapy in cancer, and significantly improved the survival and quality of life of patients. However, the main problems related to the use of antineoplastic agents in cancer treatment are still the high toxicity, low selectivity, and resistance of these drugs [3, 4]. In addition, the pharmacokinetic characteristics of antineoplastics are also limiting factors in the effectiveness of treatment, as they can trigger some problem related to absorption, distribution, metabolism and/or excretion of chemotherapy. These obstacles are responsible for serious side effects related to cancer therapy, because the risks related to the use of these drugs are generally superior to their benefits [5].
In the search for more effective and safer antineoplastic drugs, hydrazones are being widely studied for use in the pharmacotherapy of neoplasias, because they have the ability to inhibit the in vitro growth of several neoplastic cell lines, including breast, human colon, lung, esophagus, pancreas, and leukemia cancers [6]. Hydrazones have in their chemical structure the function azomethine (R-CH=N-NH) [7] and can be examples of molecular hybrids, since they are obtained through condensation reactions between biologically active aldehydes and hydrazines [8].
Cheminformatics is a useful tool in the development of new drugs with bioactivity and/ or with desired pharmacokinetic properties [9]. Among the main applications of cheminformatics in this field, the use of computational methods to filter and select potentially active compounds based on their molecular characteristics, as well as the determination of the pharmacokinetic characteristics of these compounds, stand out [10]. The use of computational tools is advantageous for the development of pharmaceuticals, since in silico screenings promote time savings, as well as financial resources [11].
In this sense a series of six hydrazones was planned by means of synthesized molecular hybridization, and their potential as new anti-cancer agents were evaluated through in vitro cytotoxicity assays against four tumor cell lines, and by in silico tests to determine the pharmacokinetic profile of the hydrazonic compounds under study.
MATERIAL AND METHODS
The hydrazones were planned and synthesized for evaluation of their antineoplastic capacities, through the evaluation of in vitro cytotoxicity against tumor cell lines. Then, to verify the potentiality of their use as drugs, the pharmacokinetic profile was evaluated by in silico tests of the series of synthesized hydrazones.
Synthesis of phenylhydrazones
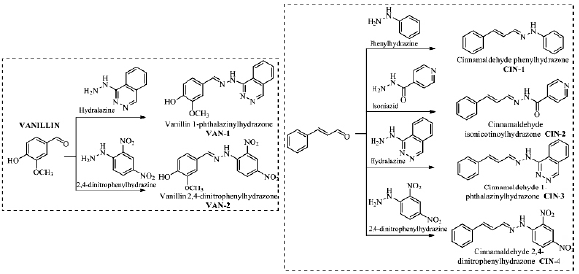
The hydrazones were obtained through the methodology described by Souza et al. [12], from the reaction between vanillin and cinnamaldehyde with hydrazines. The six hydrazones obtained were named: vanillin 1-phthalazinylhydrazone (VAN-1), vanillin 2,4-dinitrophenylhydrazone (VAN-2), cinnamaldehyde phenylhydrazone (CIN-1), cinnamaldehyde isonicotinohydrazone (CIN-2), cinnamaldehyde 1-phthalazinylhy-drazone (CIN-3), and cinnamaldehyde 2,4-dinitrophenylhydrazone (CIN-4).
In vitro cytotoxicity testing: MTT method
The cells were plated at concentrations of0.7 x 105, 0.1 x 106, 0.3 x 106 cells/mL for the HCT-116 (human colon cancer cells), HL-60 (human leukemia cells), PC-3 (human prostate cancer cells) and SF-295 (glioblastoma cells), respectively. The plates were incubated with the substance for 72 h in a greenhouse at 5.0% CO2 at 37 °C. At the end, they were centrifuged and the supernatant was removed. Then, 150 mL of MTT solution (tetrazolium salt) was added, and the plates were incubated again for 3 hours. After this period, the plates were again centrifuged to remove the MTT solution. The absorbance was read after dissolution of the formazan precipitate with 150 mL of pure DMSO in a plate spectrophotometer at 595 nm. The single concentration experiments were analyzed according to the mean ± standard deviation (SD) of the percentage of cell growth inhibition (GI %) using an OriginLab® program. To evaluate the cytotoxic potential of the samples tested, it was used the scale described by Souza et al. [13], where the hydrazones were classified as: i) no activity: GI% ≤ 0.00%; ii) low activity: GI% from 1.00% to 50.00%; iii) moderate activity: GI % from 50.00% to 75.00%; and iv) high activity: GI% greater than 75.00%.
ADME/T profile of the hydrazonic derivatives
For evaluation of the pharmacokinetic and toxicological profile (ADME/T) in silico, the program PreADMET, available online and for free at http://preadmet.bmdrc. org/, was used. The structures of the six hydrazones were designed and analyzed in the modules of prediction of ADME and toxicity. For evaluation of each parameter, the following descriptors were chosen: absorption- absorption in Caco-2 cells and human intestinal absorption (HIA); Distribution-binding to plasma protein levels (PPL) and permeability in the Blood-Brain Barrier (BBB); Metabolism-interaction with enzymes from the CYP complex; Excretion-renal excretion through permeability in MDCK cells; and Toxicity - mutagenicity, carcinogenicity in rats and cardiotoxicity by inhibition of the hERG gene. The reference values used for each parameter are described in table 1, along with the results obtained.
RESULTS AND DISCUSSION
A series composed of six hydrazones was planned by molecular hybridization using the natural aldehydes vanillin (VAN-1 and VAN-2) and cinnamaldehyde (CIN-1 - CIN-4), which are synthesized as described by Souza et al. (2020) [12] (figure 1). With the precursor vanillin, two hydrazones called VAN-1 and VAN-2, with yields of 98.0% and 70.0%, respectively, were obtained. For the cinnamaldehyde precursor, four hydrazonic derivatives were obtained, with high yields, denominated CIN-1 (91.0%), CIN-2 (84.0%), CIN-3 (X.X%), and CIN-4 (60.0%) (figure 1).
In vitro cytotoxicity test
The in vitro cytotoxicity of the respective hydrazonic derivatives (VAN-1, VAN-2, CIN-1, CIN-2, CIN-3, and CIN-4) were evaluated against four HCT-116, HL-60, PC-3 and SF-295 tumor cell lines in 72 h of the treatment. The compounds were tested at the following concentrations: 34.0; 30.1; 45.0; 39.8; 36.5 and 32.0 fxM, respectively for VAN-1, VAN-2, CIN-1, CIN-2, CIN-3 and CIN-4. And was obtaining the percentage of growth inhibition (GI%) shown for each strain, as described in figure 2.
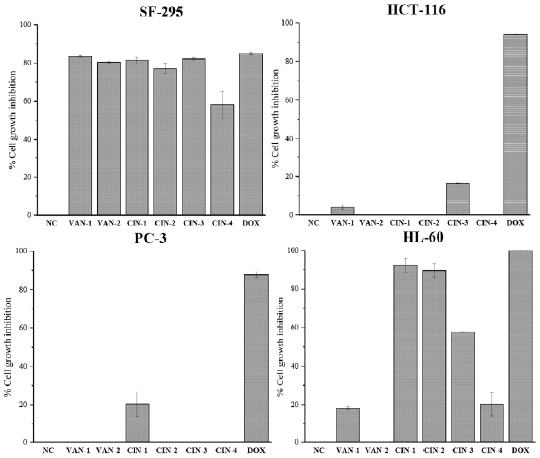
Figure 2 Cell growth inhibition percentage (%GI) of the compounds VAN-1, VAN-2, CIN-1, CIN-2, CIN-3 and CIN-4 in four tumour cell lines. Negative control (NC). Doxorubicin (DOX). Results are means ± SD.
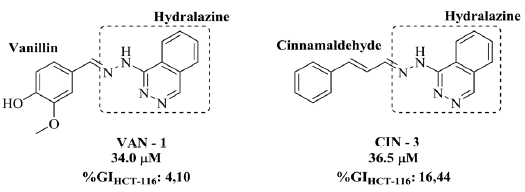
In the analysis of the results, it was observed that in front of the lineage of human colon cancerous cells (HCT-16) and prostate cancerous cells (PC-3), the tested hydrazones presented none or low cytotoxic activity (GI < 50%). In HCT-116 cells, only two derivatives presented low capacity to inhibit the growth of this cellular type, VAN-1 and CIN-3, with GI equal to 4.10 ± 1.04% and 16.44 ± 0.17%, respectively. In the specific case of these derivatives, they were obtained as a product of molecular hybridization between vanillin or cinnamaldehyde with hydralazine (figure 1), the latter a drug used in the treatment of severe hypertension, heart failure and gestational hypertension [14].
Hydralazine is a hydrazine that, in addition to its highlighted antihypertensive properties, has already shown promising antineoplastic activity against several types of cancer, such as prostate [14], leukemia [15] and cervical cancer [16]. The main mechanism of action of hydralazine in the treatment of neoplasms consists of a weak inhibition of the methylation of the DNA that is hyper-methylated during the carcinogenic process, resulting in the reactivation and expression of tumor suppressor genes [17].
Considering the antineoplastic activity already presented by hydralazine, as well as the fact that only the hydrazonic derivatives containing it in its structure presented cyto-toxicity against human colon cancer cells (HCT-116), it is possible to attribute this activity to the molecular hybridization between the phthalazinyl nucleus of hydralazine and the natural aldehydes employed, resulting in phenylhydrazones VAN-1 and CIN-3 (figure 3). Moreover, it was also found that the CIN-3 compound showed greater inhibition capacity than VAN-1, demonstrating the positive influence of the portion originating from cinnamaldehyde, with the carbon spacing between the hydra-zone function and its second aromatic ring, as well as the fact that the second ring is not replaced, resulting in a hydrazone approximately four times more active compared to HCT-116 cells.
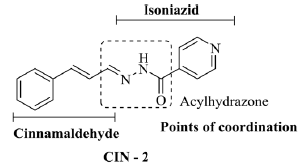
In leukemic cells (HL-60) it was observed that only the compound VAN-2 did not present cytotoxic activity, and the derivatives of cinnamaldehyde CIN-1 and CIN-2 are the most active hybrids in front of this cellular lineage, with GI of 92.37 ± 3.60% and 89.62 ± 3.54%, respectively. CIN-2 hydrazone is the only acylhydrazone in the tested series, and was obtained through molecular hybridization between cinna-maldehyde and isoniazid, and the latter is a drug used clinically in the treatment of tuberculosis [18].
The isoniazid-derived acylhydrazones present significant antitumor activity described in the literature, and this bioactive profile is mainly attributed to the chelating capacity of metals that these compounds exhibit [19]. Since neoplastic cells use iron as an essential growth factor for their proliferation [19], one of the strategies in antineoplastic pharmacotherapy is to reduce the concentration of this metal through the use of chelating substances and thus hinder the development and spread of cancer [20, 21]. The acylhydrazones derived from isoniazid, such as the compound CIN-2, present in its structure points of coordination with metals, resulting from the acylhydrazone function present in its structure that, as seen, can be related to its antineoplastic activity in relation to leukemic cells (figure 4).
As for the cytotoxic activity against prostate cancerous cells (PC-3), only CIN-1 hydrazone exhibited capacity to inhibit cell growth, with GI of 20.43 ± 6.31%, and was classified as a compound of low cytotoxic activity in relation to the scale used.
As CIN-1 is the simplest hydrazonic derivative synthesized from cinnamaldehyde, this result suggests that the modifications made in its structure have negatively influenced the cytotoxicity compared to PC-3 cells of the other derivatives, considering that none of them showed activity for this lineage.
In the tests using glioblastoma cells (SF-295), it was observed that all the tested hydrazones presented cytotoxic activity compared to this cell lineage, where only the compound CIN-4 (58.11 ± 7.03%) was classified with moderate activity due to its inhibition capacity (GI less than 75%). The other hydrazones presented high cytotoxic activity for SF-295, and increasing activities were found in the following order: CIN-2, VAN-2, CIN-1, CIN-3, and the most active VAN-1, with 83.50 ± 0.70% GI for this cell lineage.
The glioblastoma is one of the most aggressive types of cancer that affects the CNS, and like the others it has limitations in the chemotherapy treatment due mainly to its location protected by the BBB, as well as the limited therapeutic arsenal available [22]. Currently, nitrosoureas, especially carmustine (figure 5), are the main therapeutic agents used for the treatment of patients with glioblastoma, but in clinical practice, they present response rates lower than 50.0% [22, 23]; therefore, they need to be combined with other antitumor drugs. A slight structural similarity between the synthesized hydrazones and carmustine is observed, mainly in the arrangement of the nitrogen atoms present in both structures (Figure 5), which can be related to the high activity presented by these hydrazones in front of the glioblastoma lineage.
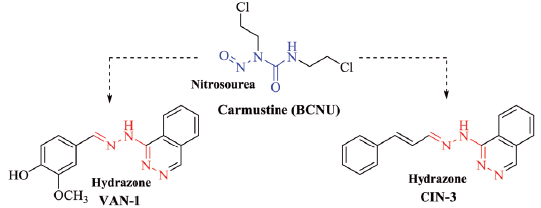
Figure 5 Structural comparison between carmustine and hydrazones was tested with higher cytotoxic activity against glioblastoma cells, emphasizing the structural similarity between the compounds.
In general, analyzing the cytotoxic activities in vitro of all hydrazones, it was observed that the compound CIN-1 was the phenylhydrazone that was more active in three of the four cancer strains tested, showing excellent bioactivity in relation to leukemia (92.37 ± 3.60%) and glioblastoma (81.53 ± 1.51%) cells. Thus, such results demonstrate the potentiality of hydrazones as antitumor, mainly against glioblastomas, the tumor cell lineage in which all the derivatives tested presented capacity of cell growth inhibition.
ADME/T profile of the hydrazonic derivatives
The pharmacokinetic parameters of absorption, distribution, metabolism, excretion and toxicity (ADME/T) of the synthesized hydrazones were evaluated using the PreADMET software in order to select the most promising hydrazone of the series as an antineoplastic drug. The prediction of the pharmacokinetic and toxicity profile of these molecules is ofparamount importance for the characterization of these compounds and for the planning of future biological trials. The in silico results obtained are described in table 1.
Table 1 Pharmacokinetic and toxicity parameters of the new molecular hybrids determined using the PredADMET software.
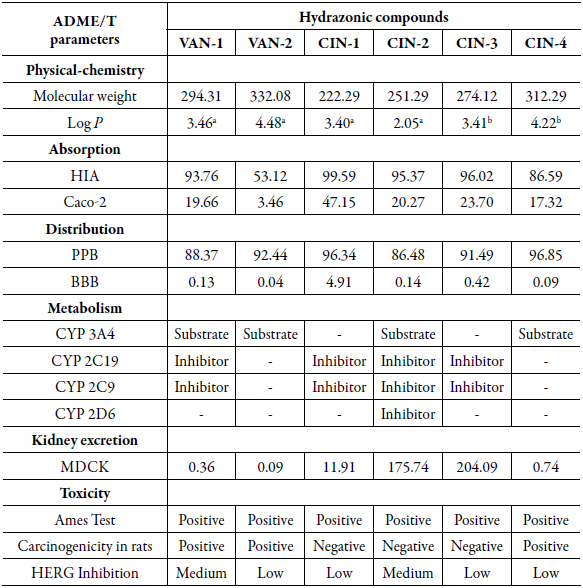
LogP: aobtained from Souza et al. [12] bcalculated in ChemDrawn®.
HIA: Low absorption 0.0-20.0%; moderate absorption 20.0-70.0%; excellent absorption 70.0-100.0% [24].
Caco2 cell permeability: High permeability >70.0 nm.sec-1; Medium permeability 4.0-70.0 nm.sec-1; Low permeability <4.0 nm.sec-1 [25];
PPB: Strong connection: greater than 90.0%; weak connection: less than 90.0% [24].
BBB: High CNS absorption: greater than 2.0; intermediate CNS absorption: between 0.1 and 2.0; low CNS absorption: less than 0.1 [24].
MDCK: Low permeability: less than 25.0 nm.sec-1; medium permeability: between 25.0 and 500.0 nm.sec-1; high permeability: greater than 500.0 nm.sec-1 [24].
Ames test: Positive: mutagenic; Negative: non-mutagenic.
Carcinogenicity: Positive: carcinogenic; Negative: non-carcinogenic.
To evaluate the absorption profile of the tested hydrazones two main prediction models were analyzed, that of permeability in cells derived from colon adenocarcinoma (Caco-2) and the percentage of human intestinal absorption (HIA). Caco-2 cells present several mechanisms for drug transport through the intestinal epithelium and are used mainly as an in vitro model to evaluate the absorption of orally administered drugs. The HIA is the sum of the bioavailability and absorption of the tested compound, evaluated from the ratio of excretion or cumulative excretion in urine, bile and feces [26].
It was observed that the majority of the tested hydrazones presented an excellent intestinal absorption rate, when the AHI percentage was analyzed. It was verified that only the VAN-2 compound presented intermediate absorption when analyzed in this in silico model (20% < HIA < 70%). This high rate of intestinal absorption reflects in a high bioavailability of the drug after oral administration, and this is one of the greatest challenges in the development of new antineoplastic [27].
The high absorption of the tested hydrazones is related to their Log P values (2.05 -4.48) which, when compared to their precursors, cinnamaldehyde (Log P 1.67) and vanillin (Log P 1.27), proved to be superior. Higher Log P values obtained for hydrazones indicate greater lipophilicity of these compounds than their precursors, and this fact probably results from the presence of hydrazone function [28], resulting in better absorption by biological membranes.
Therefore, the hydrazone with the best absorption profile in the analyzed model was CIN-1, with HIA equal to 99.59% and permeability in Caco-2 cells equal to 47.15 nm.sec-1. These data can be derived from its structural simplicity considering that it is the hydrazone with the lowest molecular mass (222.29 g.mol-1) and that does not present ionizable groups in its structure. Such structural and physical-chemical characteristics allow the CIN-1 compound to diffuse more easily through the biological membranes, when compared to the other derivatives of the analyzed series, besides the other transport mechanisms common to the other hydrazones.
For a prediction of the distribution profile of the new hydrazones the ability to bind to plasma proteins (PPL) and the ability to cross the BBB were evaluated. The degree of PPL is of utmost importance for a drug candidate, since the process of its distribution by the body affects the availability, action and consequent efficacy of the drug, and it should be considered for the determination of the appropriate dosage [29]. Thus, it was found that most of the hydrazones analyzed had high affinity for PPL (92.4499.59%); however, only VAN-1 and CIN-2 showed weak bonding in silico tests.
For a good in vivo distribution it is considered that the pharmacological effect of the drug is promoted by molecules that are not linked to the carrier proteins [30], since in the free form, such molecules can spread through biological membranes and reach their specific targets [31]. Thus, for a good distribution it is necessary that the drug does not interact so strongly with the plasmatic proteins, so that this connection is easily undone in order to connect it to its pharmacological target. Thus, it can be observed that VAN-1 and CIN-2 presented the desirable distribution profile by the peripheral circulation, so they can perform their cytotoxic functions satisfactorily.
Penetration through BBB is an important pharmacokinetic parameter for the analysis of potential drugs to be considered for the treatment of CNS diseases [24, 29]. Most of the hydrazones tested showed high cytotoxicity compared to glioblastoma cells (table 1), and there is great interest in knowing the BBB absorption profile of these new compounds. Among the hydrazones analyzed, it was observed that CIN-1 presented the highest rate of absorption by BBB, as well as high capacity to inhibit the growth of glioblastomas in vitro (CI 81.53 ± 1.51%). Therefore, CIN-1 hydrazone proved to be more promising as a specific antineoplastic agent for glioblastomas. We know that the in silico distribution characteristics suggest that, in vivo, this derivative is able to reach the glia cells and produce the desired cytotoxic effect in the treatment.
The metabolism of hydrazones was evaluated by the ability to inhibit four enzymes of the cytochrome P450 complex (CYP450), which consists of a family of hepatic enzymes responsible for the metabolism of endogenous and xenobiotic substances in the human body [11]. CYP450 is responsible for the metabolism of approximately 90% of xenobiotics in the human body [32-33], including drugs, which is mainly performed by the CYP3A4, CYP2C19, CYP2C9 and CYP2D6 [34]. The inhibition of some of these enzymes indicates the potentiality of the drug tested to interact with the metabolism of other drugs that use this same route of biotransformation, making them accumulative and, consequently, toxic to patients [25, 34, 35].
Therefore, it was observed that for the series of hydrazones analyzed only the compounds VAN-2 and CIN-4 were not shown as potential inhibitors of the tested CYP isoforms, as they are potential substrates for CYP3A4 only. This is an ideal characteristic for a potential antineoplastic drug, since chemotherapy for cancer treatment is usually polymedicamentous [36].
The inhibition of some enzyme of the CYP complex can also be correlated with the molecular mass ofxenobiotics, so that molecules with lower molecular masses can inhibit more effectively the enzyme by facilitating access to allosteric sites [25]. Observing the in silico capacity of CYP inhibition by the analyzed hydrazones, only VAN-2 and CIN-4 were not able to inhibit any of the CYP isoforms, because they are the molecules with higher molecular masses, 332.27 and 312.29 g.mol-1, respectively.
For analysis of the excretion profile of hydrazones, the MDCK cell permeability model, used to predict the excretion of drugs and other chemicals, was evaluated [24]. Most hydrazones had low permeability to MDCK cells in silico, suggesting that these hybrids would have high time of excretion by renal route [30]. In the series of compounds, only CIN-2 and CIN-3 had intermediate permeability.
The toxicity of antineoplastic compounds should also be analyzed, especially parameters such as carcinogenicity, mutagenicity and cardiotoxicity, since most commonly used antineoplastic drugs are mutagenic and teratogenic in in vivo tests (animal models) [37]. The model for predicting the capacity of hydrazones to be mutagenic was the Ames test, which was developed in 1972 and which makes use of strains of Salmonella typhimurium bacteria, used to evaluate whether the compounds tested can generate damage to bacterial DNA [38, 39]. In this model, it was verified that all the hydrazones tested were shown as potential mutagenic agents, and this action may be related to cytotoxic activity [40] compared to cancer cell lines presented by these hybrids. Thus, further in vitro and in vivo studies should be carried out in order to prove the mutagenicity and verify whether the therapeutic benefits of these hydrazones outweigh the risks of their administration [41].
Carcinogenicity should also be a parameter evaluated in the toxicity of antineoplastics, since many of these compounds commonly used in clinical practice have been associated with the development of secondary cancers in treated patients [37, 42, 43]. For the hydrazones analyzed it was found that three of the six derivatives tested in silico showed no carcinogenic activity in rats, these being the derivatives of cinnamaldehyde CIN-1, CIN-2 and CIN-3, indicating a relevant safety rate in this parameter.
The cardiotoxicity of antineoplastics is one of the most devastating sequels of cancer therapy [43] and should also be evaluated in the development of new antineoplastics. The inhibition of the human gene homologous to ether-a-go-go (HERG) results in compromised expression of the HERG channels (cardiac channels of K+) and the consequent appearance of cardiac problems, in some fatal cases, caused by the administration of the drug [39]. Thus, the toxicity evaluation of new antineoplastic agents must take into consideration, among other parameters, the action of drugs on the HERG gene. Thus, it was observed that the tested hydrazones present an intermediate to low risk in inhibiting the expression of the HERG gene and, consequently, they are not potentially cardiotoxic derivatives according to the in silico tests.
After evaluation of the pharmacokinetic profile of the six synthesized hydrazones, it was observed, in a general way, that the hydrazones present promising characteristics of intestinal absorption, distribution, metabolism, excretion, and toxicity. As antineoplastic multi-target drugs, the hydrazones derived from cinnamaldehyde, the compounds CIN-1 and CIN-2, which showed high cytotoxic activity compared to leukemia cell lines (HL-60) and glioblastomas, stand out (SF-295). In addition, it was observed that CIN-2 presented the most promising ADME/T in silico profile, showing high intestinal absorption, desirable distribution profile related to plasma protein binding, good renal excretion and low toxicity. In addition, the ADME/T profile of CIN-1 highlights its use as a promising antineoplastic agent with action in the CNS, more specifically in the treatment of glioblastomas.
Among the series analyzed, it was observed that the hydrazones derived from cinna-maldehyde, CIN-1 and CIN-2, presented high cytotoxic activity in vitro compared to two (HL-60 and SF-295) of the four tested cancer cell lines.
When analyzing the ADME/T profile, it was observed that the hybrid CIN-2, the only acylhydrazone of the synthesized series, obtained, in general, better pharmacokinetic characteristics, with emphasis on absorption, distribution, renal excretion and toxicity, and was the most promising hydrazone of the series.