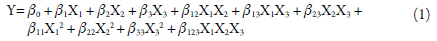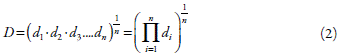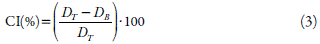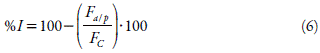The species Calophyllum brasiliense Cambés (Calophyllaceae) is widespread through out Brazil, growing in all kinds of environments from humid climates such as the Amazonia and dries one like Brazilian Cerrado [1]. This species is popularly known as jacareúba [2]. The tree reaches about 40 m in height, 3 m in diameter with a thick stem bark (≈2 cm). The infusion of the stem bark of Calophyllum brasiliense Cambés (CBC) is used for treating infected wounds, stomach pains, liver disorder, inflammation, dia betes, diarrhea, and hemorrhoids [3]. Despite the great pharmacological potential of this medicinal plant, few efforts have been made for transforming the stem bark of CBC and its extracts in standardized products that can be used, safely, in therapeutic.
The ethanolic extract of the stem bark (CBE) content phenolics and flavonoids compounds as gallic acid, quercetin, and hyperin [1]. Also content xanthones and couma-rins [4, 5]. The anti-inflammatory and hypoglycemic effect of CBE has been associated with phenolics and flavonoids [6]. The high electronic density of phenolics and fla-vonoids induces strong scavenging of reactive oxygen species (abundant in decom-pensated diabetics), contributing to the patient homeostasis [7]. On the other side, phenolics and flavonoids are strong inhibitors of enzymes associated to protein and carbohydrate metabolism [8], contributing indirectly to lowering the blood glucose of diabetics [3, 9, 10].
The stability of phenolics and flavonoids compounds in solution is affected by factors such as light, temperature, oxygen, and pH [11]. CBE and its hydroalcoholic solutions when unprotected from light under ambient conditions develop a superficial and firm film that is thicker as the concentration of solids increase. This probably occurs by the oxidative effect of light and/or oxygen on xanthones and phenolics. For this reason, the preparation of a dried powder could be an excellent way to preserve and stabilize the actives present in the extract. Dried extracts present advantages as compared with liquid extracts such as a higher concentration, facility of storage, and transportation, and others [12].
Among the techniques for drying vegetal extracts, spray drying appears as the most promissory, allowing to prepare solid products from fruits, vegetables, and medicinal plant at a relatively low cost with additional benefices to final products, like a better stability, improved flowability and compressibility [12]. Vegetal extracts to be dried by spray drying are added with drying adjuvants, which allow obtaining powders with low moisture content, avoiding active degradation, and suitable physic mechanical properties, which is particularly important for pharmaceuticals formulations.
Metabolites present in plant extracts can activate several physiologic responses at the same time. For this reason, the use of plant extracts in diabetics arises as an excellent possibility of treatment. Thus, this work aimed the optimization of drying parameters for obtaining the spray-dried extract of Calophyllum brasiliense Cambés (SDE), and the evaluation, in vitro, various antidiabetic mechanisms (antioxidant, antiglycant, gli-cosidase inhibition). The hypoglycemic effect of the SDE was also evaluated in Wistar rats, in an alloxan-induced diabetic model.
MATERIALS AND METHODS
Vegetal material
The stem bark of Calophyllum brasiliense was collected from a population of adult trees located at 0°87'16.8''N, -51°14'62.6"W, Municipality of Ferreira Gomes, Amapá, Brazil. The species were identified, and the voucher (code 0598AP) was deposited in the Herbarium of the Institute of Studies and Research of the Amapá State (Iepa), Brazil. The drug was dried in the shadow to a constant moisture content (<14%) and milled using a knife mill, for obtaining a particle size «5 mm.
Extract preparation
CBE was prepared by dynamic maceration. The drug (500 g) was placed in an amber bottle containing 2L of 70 % hydroalcoholic solution for 72 h. The mixture was mechanically stirred for one hour, twice a day, for three days. The extractive solution was filtered using a cotton cloth and concentrated in a rotary evaporator (Ika® Werke GMBH & Co., Germany) at 40 °C, up to a final drug: solvent ratio of 2:1 (g/mL). The extract was placed into an amber bottle and stored at room temperature, protected from light.
Extract characterization
Immediately after the preparation, the appearance, aroma, and flavor of the CBE were evaluated. Total solids, density, and the refraction index were also evaluated [13]. The pH was directly measured using a pH-meter (Tecnopon®, Brazil), previously calibrated with buffer solutions (pH 4, and 7, Alphatec®, Brazil). For the development of the experimental design, the total phenolic content was determined by the Folin-Ciocal-teu spectrophotometric method, using gallic acid (Sigma®, USA) as the standard [14]. The content of gallic acid in the SDE was determined by HPLC (Sigma®, USA). Fla-vonoids content was determined spectrophotometrically by the aluminum chloride method, using a quercetin primary standard (Sigma®, USA) [14]. All measurements were performed in triplicate and expressed as the mean with the standard deviation.
Adjuvants selection
Three experiments were made for selecting the drying aids to be used for preparing the spray-dried extract of Calophyllum brasiliense (SDE). The CBE (100 mL) was added with 0.25 percent (w/v) of colloidal silicon dioxide plus 5 g of microcrystalline cellulose (Avicel® PH 102), or lactose monohydrate (Sigma®, USA), or maltodextrin (Sigma®, USA). The following drying conditions were used: air drying speed 4.0 m3/h, feed flow rate 8 mL/min, an inlet air temperature of 110 °C. The adjuvants that allow obtaining the dried product with the highest phenolics content, the highest yield, and the lowest moisture content will be selected.
Drying process
For preparing the SDE, a Mini-Spray Dryer (MSD) 1.0 (Labmaq®, Brazil) was used. A D-optimal experimental design was applied for optimizing the drying conditions (table 1). The operational parameters used as factors were air drying speed (ADS, 3.5-4.5 m3/h), the feed flow rate of the suspension (FR, 5-11 mL/min), and the inlet air temperature of the drying air (IAT, 90-130 °C). The airflow for spraying the suspension into the drying chamber (30 L/min), and the nozzle diameter (1.0 mm) were kept constant.
Table 1 Outcomes of the D-optimal experimental design for the drying optimization showing the factors in actual and coded values.
| Run | Inlet air temperature (°C) | Feed flow rate (mL/min) | Air drying speed (m3/min) |
|---|---|---|---|
| 1 | 130 (+1) | 5 (-1) | 3.5 (-1) |
| 2 | 130 (+1) | 11 (+1) | 3.5 (-1) |
| 3 | 90 (-1) | 11 (+1) | 4.5 (+1) |
| 4 | 130 (+1) | 5 (-1) | 0.5 (+1) |
| 5 | 90 (-1) | 5 (-1) | 0.5 (+1) |
| 6 | 130 (+1) | 11 (+1) | 0.5 (+1) |
| 7 | 110 (0) | 8 (0) | 4 (0) |
| 8 | 90 (-1) | 11 (+1) | 3.5 (-1) |
| 9 | 110 (0) | 8 (0) | 4 (0) |
| 10 | 90 (-1) | 5 (-1) | 3.5 (-1) |
| 11 | 110 (0) | 11 (+1) | 3.5 (-1) |
| 12 | 110 (0) | 5 (-1) | 3.5 (-1) |
| 13 | 130 (+1) | 8 (0) | 4 (0) |
| 14 | 110 (0) | 11 (+1) | 4 (0) |
| 15 | 110 (0) | 8 (0) | 5 (+1) |
| 16 | 90 (-1) | 8 (0) | 4 (0) |
| 17 | 110 (0) | 5 (-1) | 4 (0) |
| 18 | 110 (0) | 8 (0) | 3.5 (-1) |
| 19 | 90 (-1) | 5 (-1) | 4.5 (+1) |
| 20 | 110 (0) | 8 (0) | 4 (0) |
| 21 | 130 (+1) | 5 (-1) | 0.5 (+1) |
| 22 | 90 (-1) | 5 (-1) | 3.5 (-1) |
| 23 | 110 (0) | 8 (0) | 4 (0) |
Coded values in parenthesis: high-level (+), center (0), low-level (-1).
The suspension was stirred during the drying process using a magnetic stirrer (Fisaton®, Brazil). Phenolics content (%), process yield (%), the water activity, outlet temperature (°C), and the moisture content (%) were used as responses. The response outcomes were fitted to mathematical models (equation 1) using the Design Expert Software 12.0 (StatEase®, USA). The effect of factors for constructing the theoretical models was estimated using the Backward elimination technique. In this technique, all factor is entered into the first model equation, and each one is eliminated (one at a time) if they do not contribute (p-value >0.05) to the model fitting [15].
Where Y are the responses and β are the coefficients representing the magnitude of the factors' effect. β 0, intercept; β 1, β 2, β 3 are the linear effect of factors X1, X2 X3; β 12, β 13, β 23 are the effect of interaction of the factors X1X2, X1X3, X2X3; β 11, β 22, β 33, the effect of the quadratic factors X1, X2 X3; and β 123 are the effect of the interaction of the factors X1X2X3.
The optimization of the drying process was performed using the Desirability function, a multiple response approach [15]. This function allows assigning levels of importance to responses for signaling the desirable value, to achieve the optimized response (equation 2):
However, the predictive power of the Desirability function (D) depends on the predictive power of the adjusted model of the individual responses. Thus, the high the good ness of fit (R2) and goodness prediction (Q2) of the adjusted model the higher the predictor capability of the Desirability function. Desirability represents the geometric mean of all transformed responses, being d i the desirable ranges of the response i; and n, the number of responses used for optimization.
Water activity
The water activity of the SDE was determined using a water activity meter (Aqua Lab 4Tev®, USA). The assay was made in triplicate, and the result was expressed as the mean ± standard deviation.
Moisture content
Moisture content of SDE was performed immediately after the dried process using an Infrared Moisture Analyzer (Shimadzu®, Japan). The assay was made in triplicate.
Dry extract flowability and compressibility
Carr's index (CI) was determined as Lachman and co-workers [16]. For determine the Carr's index, the bulk (D B ) and taped (D T ) density were determined using a powder flow analyzer (Micro-System®, UK). CI was calculated as equation 3:
Flow rate (F R ) was measured by pouring a mass (m) of SDE in a glass funnel (wall of 45°) with an exit orifice of 8.0 mm and measuring the time (t) taken for the mass powder to flow through the orifice. The flow rate was calculated using equation 4:
Scanning electronic microscopy
Particle morphology was evaluated by Scanning Electronic Microscopy (SEM) (Hitachi® TM3030 Plus, Japan). A sample of the dry extract was spread out over the microscopy sample slide using a carbon adhesive tape and directly positioned on the microscope chamber. Microphotographs were taken using an accelerating voltage of 5.0 kV, and a magnification of 1000, and 5000x.
Particle size analyses
The Sauter Mean Diameter (D3,2) and the particle size distribution were determined by optical microscopy, using an Olympus® microscopy (USA). Images of the SDE (thoughtfully dispersed in a glass lamina) were taken using a Canon® camera ( Japan). Images were analyzed using Image Pro-plus 7.0® Software. A total of 1500 particles measured, and the particle size and the size distribution were determined using Stat-Graphics XV. 1 (Stat Easy®, USA).
DPPH radical scavenging
The radical scavenging activity was evaluated by spectrophotometry (Shimadzu®, Japan), using the DPPH (1,1-diphenyl-2-picrylhydrazyl) radical [10, 17, 18]. For biological assays, the blank solution was prepared by dissolution of 0.25 g of colloidal silicon dioxide plus 5 g of lactose monohydrate in 100 mL of PBS buffer (pH 7.4). The mixture was sonicated for 10 min, filtered by a Millipore membrane (0.45 fzm), and diluted at the same concentrations used for the SDE (2.5, 5, 10, 15, 20, 25, 50 and 100 μg/mL).
Alpha-glycosidase inhibition
The assay was made in 96-wells microplates according to Kiho and coworkers [17, 19]. The enzyme solution was prepared by dissolving 3 mg of rat intestinal acetone powder (Sigma®, USA) in 1 mL of buffer phosphate (pH 6.9). The solution was centrifuged at 5000 rpm for 15 min, filtered by a Millipore® membrane (0.45 fzm), and the supernatant was used for the assay. The standard and the tested substances were prepared in concentrations of 2.5, 5, 10, 15, 20, 25, 50, and 100 μg/mL. In the microplate wells were placed 30 μL of DMSO (used as the control), the standard solution of Acarbose®, and the SDE solutions. To each well was added 170 μL of enzyme solution. Microplates were incubated protected from light, for 5 min, at 37 °C. After that, 100 μL of 4-nitrophenyl a-d-glucopyranoside was added to each well and incubated again for 20 min. The analysis of the percent of inhibition was performed in triplicate and it was determined using equation 5:
A a/p = Absorbance of the Sample-Absorbance of the blank, A C = Absorbance of the negative control-Absorbance of the Blank.
The blank solution was prepared as described above for evaluating the antioxidant effect. The IC50 values were estimated by Probit analysis using the GraphPad® Software (USA), at a level of significance of p=0.05.
Pancreatic lipase inhibition
The assay was made in 96-wells microplates according to Kiho and coworkers [17, 19]. The enzyme solution was prepared by dissolving 4 mg of pancreatic lipase (type II, L3126-25G, Sigma®, USA) in 5 mL of Trisma-HCl (pH 7.6). After that, the substrate solution was prepared by dissolving 20 mg of 4-nitrophenyl palmitate (N2752-50G, Sigma®, USA) in 5 mL of acetonitrile: ethanol (1:1) solution. The standard substance, the blank (prepared as described before), and the SDE were prepared in concentrations of 2.5, 5, 10, 15, 20, 25, 50, and 100 μg/mL. The wells were prepared as follows: 30 μL of the DMSO (Control), Orlistat® (Standard drug), and the SDE solutions. After that, each well was added with 250 μL of the enzymatic lipase solution, and the microplates were incubated in the dark for 5 min, at 37 °C. After that, 20 μL of substrate solution was added to each well. Microplates were incubated again for 10 min until the absorbance of the Control (at 450 nm) was equal to 1.00 ± 0.10. The assay was performed in triplicate, and the percent of inhibition was calculated by using the equation 5 again. The IC50 was estimated by Probit analysis using the GraphPad Software (USA), at a level of significance of p=0.05.
Antiglycation activity
Oxidative path way
The assay was made according to Kiho and co-workers with modifications [17]. A reaction mixture was prepared to afford 10 mL of a buffer phosphate solution (0.20 M, pH 7.4) containing BSA (10 mg/mL), glyoxal (30 mM), and sodium azide (3 mM) as an antibacterial agent. For the assay, 300 μL of the reaction mixture was incubated with solutions of different concentrations of SDE (10, 25, 50, 75, 100 μg/mL, gallic acid equivalent). Quercetin was used as the standard antiglycant oxidative agent and DMSO as the negative control. As a blank, a phosphate buffer saline solution (100 μL) was used. Microplates containing substances to be evaluated were incubated at 37 °C, for 72 h, in darkness. After that, the fluorescence intensity at 370 nm (excitation) and 447 nm (emission) using a spectrofluorometer RF-1500 (Shimadzu®, Japan). The assay was made in triplicate. Results were expressed as a percent of inhibition for estimating the IC50, using the statistic package GraphPad Prism® 6.0, USA. Percent of inhibition was calculated as equation 6:
F a/p is the blank fluorescence less the sample fluorescence, and F C is the blank fluorescence less the control fluorescence.
Non-oxidative path way
The assay was made in the same conditions used for the oxidative pathway but using fructose (0.1 mM) instead glyoxal. Aminoguanidine was used as the standard antiglycant drug by a non-oxidative pathway [17].
Hypoglycemic activity
Forty-nine female albino rats (10-12-week-old) were acclimatized for a week, under controlled temperature (22 ± 3 °C), relative humidity (60 ± 10%), and 12/12 h light-dark cycle. They had free access to chow and distilled water during the experiment. The assay was performed in agreement with the Ethics Committee for Use of Laboratory Animals (Ceua). Federal University of Amapá (Unifap), Macapá-AP, Brazil, protocol number 012/2017. Alloxan (100 mg/kg/every 72 h, for a week) was used by the intraperitoneal route for inducing diabetes. The blood glucose was determined using a standard glucometer. Animals with glucose level above 250 mg/dL were randomly assigned to six experimental groups (7 animals each, group II to VII):
Group I, healthy group, treated with one mL of distilled water.
Group II, diabetic group, received one mL of distilled water.
Group III, diabetic group, received daily, glibenclamide 25 mg/kg.
Group IV, diabetic group, received daily, 5 mg/kg of SDE.
Group V, diabetic group, received daily, 25 mg/kg of SDE.
Group VI, diabetic group, received daily, 50 mg/kg of SDE.
Group VII, diabetic group, received daily, 100 mg/kg of SDE.
Doses of SDE were calculated as gallic acid equivalent. Treatments were administered (orally) for 25 days, using an intragastric cannula. The blood glucose was determined at days 0, 5, 10, 15, 20, and 25 using a standard glucometer (Roche®, Germany). At the same time, the food and water intake, the body weight, and the total serum cholesterol and triglycerides were determined using standard procedures of commercial kits (Sigma®, USA).
RESULTS
Physic-chemical properties of CBE
The physic-chemical properties of the fresh extract of Calophyllum brasiliense are presented in table 2.
Table 2 Physicochemical properties of the extract of Calophyllum brasiliense Cambés (n=3).
| Property | Value |
|---|---|
| Appearance | Brownish liquid |
| Aroma | Woody odor |
| Flavor | Astringent and bitter |
| Total solids (%) | 2.84 ± 0.25 |
| Density at 25°C (g/mL) | 0.987 ± 0.012 |
| pH | 5.49 ± 0.02 |
| Refraction index (n D at 25 °C) | 1.543 ± 0.025 |
| Flavonoid (as quercetin (%)) | 0.65± 0.12 |
| Gallic acid (%) | 1.27 ± 0.10 |
Drying process
Table 3 presents characteristics of the dry products obtained using different combinations of drying adjuvants. The process performed with lactose monohydrate combined with colloidal silicon dioxide produced the dried extract with the higher phenolic content and yield, and the lesser moisture. Based on these results, lactose monohydrate (5%), and colloidal silicon dioxide (0.25%), were selected as drying aids for preparing the spray dried extract. The outcomes of the experimental design for the drying optimization process are presented in table 4.
Table 3 Properties of dry products obtained using different drying aids (n=3).
| N.° | Drying aids (%) | Yield | Phenolics | Moisture | |||
|---|---|---|---|---|---|---|---|
| CDS | MCC | LM | MD | (%) | (%) | (%) | |
| 1 | 0.25 | 5.00 | - | - | A67.74±2.19 | A 5.35±0.19 | A 8.30±0.42 |
| 2 | 0.25 | - | 5.00 | - | B75.53±3.15 | B 6.37±0.33 | B 5.62±0.38 |
| 3 | 0.25 | - | - | 5.00 | B76.11±2.35 | C 4.86±0.72 | C 8.87±0.16 |
CSD: colloidal silicon dioxide; MCC: microcrystalline cellulose; MD: maltodextrin. Different letter in a column indicates statistically significant differences.
Table 4 Outcome's matrix of the experimental design for optimizing the drying process of the hydroalcoholic extract of Calophyllum brasiliense (n=3).
| Run | Phenolic Content (%) | Process Yield (%) | Water activity | Moisture Content (%) | Outlet Temperature (°C) |
|---|---|---|---|---|---|
| 1 | 5.61 ± 0.23 | 66.84 ± 2.33 | 0.47 ± 0.07 | 5.72 ± 0.42 | 105.00 ± 1.25 |
| 2 | 5.93 ± 0.19 | 66.21 ± 3.11 | 0.47 ± 0.07 | 4.86 ± 0.12 | 93.00 ± 1.33 |
| 3 | 5.27 ± 0.22 | 77.97 ± 0.98 | 0.58 ± 0.09 | 5.18 ± 0.24 | 63.60 ± 1.42 |
| 4 | 5.42 ± 0.28 | 57.38 ± 1.55 | 0.43 ± 0.08 | 5.21 ± 0.29 | 112.70 ± 1.88 |
| 5 | 5.42 ± 0.20 | 71.48 ± 2.22 | 0.44 ± 0.09 | 4.95 ± 0.22 | 78.70 ± 1.95 |
| 6 | 4.84 ± 0.17 | 77.71 ± 3.47 | 0.51 ± 0.08 | 6.87 ± 0.31 | 100.00 ± 1.22 |
| 7 | 5.17 ± 0.19 | 70.63 ± 2.10 | 0.57 ± 0.07 | 5.78 ± 0.27 | 85.60 ± 1.37 |
| 8 | 6.13 ± 0.31 | 71.79 ± 2.99 | 0.49 ± 0.08 | 6.13 ± 0.41 | 64.00 ± 1.38 |
| 9 | 5.71 ± 0.23 | 74.74 ± 2.46 | 0.43 ± 0.09 | 6.75 ± 0.40 | 82.00 ± 1.76 |
| 10 | 4.97 ± 0.17 | 74.87 ± 2.45 | 0.57 ± 0.06 | 5.91 ± 0.09 | 73.00 ± 1.19 |
| 11 | 7.89 ± 0.21 | 70.31 ± 0.75 | 0.40 ± 0.09 | 5.03 ± 0.19 | 87.30 ± 1.99 |
| 12 | 6.06 ± 0.19 | 68.44 ± 1.21 | 0.68 ± 0.05 | 5.61 ± 0.23 | 90.67 ± 1.28 |
| 13 | 6.26 ± 0.23 | 73.87 ± 4.51 | 0.38 ± 0.09 | 5.57 ± 0.26 | 107.67 ± 1.72 |
| 14 | 6.22 ± 0.22 | 74.66 ± 1.77 | 0.62 ± 0.08 | 5.27 ± 0.29 | 88.33 ± 1.55 |
| 15 | 6.97 ± 0.19 | 64.57 ± 2.85 | 0.53 ± 0.05 | 7.29 ± 0.38 | 91.00 ± 1.63 |
| 16 | 5.38 ± 0.22 | 58.47 ± 0.88 | 0.53 ± 0.03 | 6.58 ± 0.33 | 70.70 ± 2.51 |
| 17 | 6.33 ± 0.10 | 70.01 ± 1.71 | 0.41 ± 0.03 | 6.64 ± 0.22 | 91.00 ± 1.79 |
| 18 | 7.17 ± 0.18 | 73.74 ± 2.08 | 0.57 ± 0.08 | 6.54 ± 0.28 | 83.00 ± 1.82 |
| 19 | 5.62 ± 0.11 | 72.98 ± 3.55 | 0.43 ± 0.06 | 5.12 ± 0.15 | 70.50 ± 1.80 |
| 20 | 5.26 ± 0.22 | 72.13 ± 2.82 | 0.44 ± 0.09 | 5.80 ± 0.19 | 87.20 ± 1.49 |
| 21 | 5.75 ± 0.29 | 58.88 ± 1.69 | 0.44 ± 0.09 | 5.34 ± 0.25 | 105.36 ± 1.65 |
| 22 | 5.21 ± 0.20 | 73.37 ± 2.31 | 0.56 ± 0.09 | 5.72 ± 0.27 | 72.25 ± 2.23 |
| 23 | 6.01 ± 0.16 | 73.24 ± 3.21 | 0.44 ± 0.09 | 6.35 ± 0.30 | 90.25 ± 1.28 |
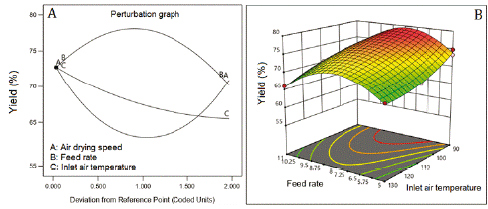
Inlet air temperature and the feed flow rate were the factors that most affected phenolic content (figure 1A). The highest phenolic content was reached at 110 °C (Central level of the inlet air temperature). The increase in the feed flow rate of the suspension improved the phenolic content being higher at 11 mL/min. The air-drying speed practically no affects the phenolics content. The data of this response was transformed into the inverse square root (1/√) for fitting a quadratic model that showed a non-significative Lack of fit (table 5), adjusted R2 of 0.8237, and Q2 of 0.7888. figure 1B represents the combined effect of factors on phenolic content, showing the large curvature of the response surface, due to the quadratic effect of the factors and their interactions (table 5).
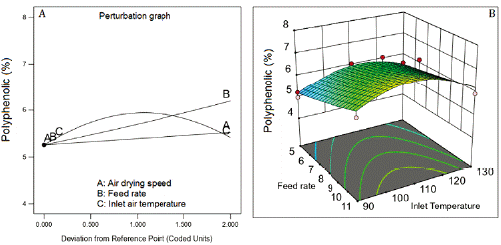
Figure 1 Effect of feed flow rate and inlet air temperature on phenolics content. Air drying speed at 4 m3/h.
Table 5 Effect of factors and interactions and their contribution to fitting the theoretical models of responses.
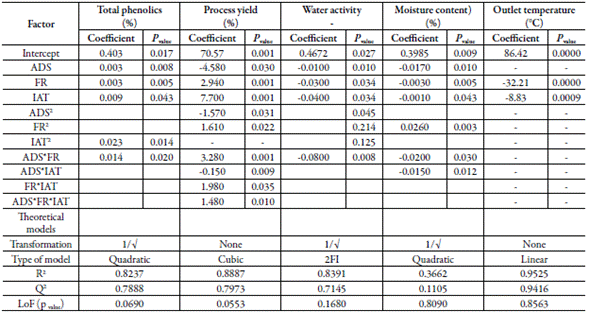
Table 5 also presents the effect of factors and their interactions on SDE properties. Mathematical transformations were made (when possible) for fitting the response outcome to theoretical mathematical models.
The feed flow rate of the suspension was the factor that most affected the performance of the drying process (figure 2A). The highest yield was achieved at a feed flow rate of 8 mL/min (center level), being minor at the lower (5 mL/min) and higher (11 mL/min) levels. The increase of inlet air temperature (line C, figure 2A) and the air-drying speed (line A, figure 2A) worsened the yield. The outcome of yield was fitted to a reduced cubic model with an adjusted R2 of 0.8887 and Q2 of 0.7973. Figure 2B shows the large curvature of the response surface caused by the effect of factors and their interaction (table 5). The interactions feed flow rate: air-drying speed and feed flow rate: inlet air temperature improved the yield. However, the interaction air-drying speed: inlet air temperature diminished the yield (coefficients with negative signs, table 5).
Water activity and moisture content
Water activity and the moisture content showed inverse correlation with the three factors (figure 4A and 5A). The inverse square root of both responses fitted quadratic models. The fitted model for water activity showed good predictability (table 5) with a difference of 1.246 between R2 (0.8391) and Q2 (0.7145). Contrarily, the model fitted for moisture content showed low values of R2 (0.3662) and Q2 (0.1105), which means low predictability. The response surface graph ofwater activity (figure 3B) shows a decreasing curvature produced by the interaction between the air-drying speed and the feed flow rate (coefficient with a negative sign, table 5). Figure 4B presents the sharp curvature of the moisture content produced by the quadratic effect of feed flow rate (a positive sign of the coefficient FR2, table 5), which was maximum at 8 mL/min (center level).
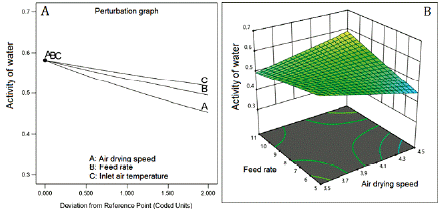
Figure 3 Effect of feed flow rate and air-drying speed on water activity. Inlet air temperature at 110 °C.
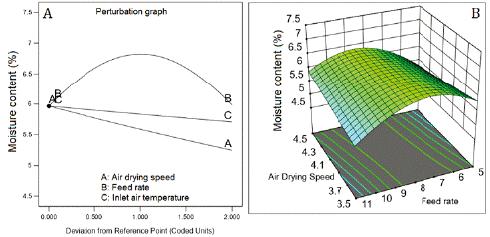
Figure 4 Effect of feed flow rate and the air-drying speed on moisture content. Inlet air temperature at 110 °C.
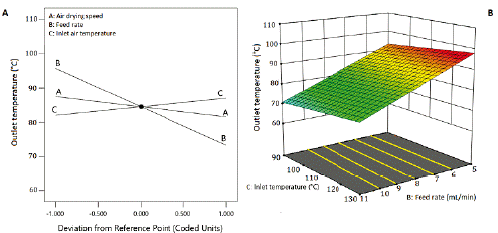
Figure 5 Effect of inlet air temperature and the feed flow rate of the suspension on the outlet temperature. Air drying speed at 4 m3/h.
The outlet air temperature diminished as the feed flow rate increased, showing an inverse correlation (line B, figure 5A). The outlet air temperature raised with the increase of inlet air temperature. By their side, as the air-drying speed increased, the outlet temperature decreased, showing an inverse correlation. This response fits a linear model with an adjusted R2 of 0.9525, Q2 of 0.9416, and a non-significant Lack of fit (table 5). Figure 5B shows a sharp decrease in the response surface of the outlet air temperature caused by the linear effect of the factors, but mainly, by the effect of the feed flow rate.
Multiple response analyses
The optimization of the drying conditions was made by using the Desirability function. The objective was to obtain a dried extract with the high phenolics content, with an excellent product recovery (yield), a low moisture content, and a low activity of water. The prediction showed a Desirability value of 0.731. The predicted value for each parameter is presented in figure 6.
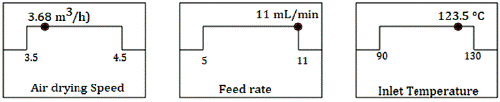
Figure 6 Values of the factors predicted by the desirability function for optimizing the spray drying process.
Three experiments were made using for drying the predicted conditions: Air Drying Speed 3.68 m3/h, Feed flow rate 11 mL/min, and Inlet air temperature 123.5 °C. Table 6 presents a comparison between the predicted and experimental results at p <0.05. In all cases, the t-test suggests that there were no statistically significant differences between the experimental and the predicted values.
Table 6 Statistical comparison of Predicted vs. Observed results for the drying optimization process.
| Response | Predicted value | Observed value | t- test, p-value |
|---|---|---|---|
| Yield (%) | 74.91 ± 2.60 | 72.75 ± 1.60 | 0.0729, 0.9454 |
| Phenolic content (%) | 6.48 ± 0.62 | 6.97 ± 0.05 | 1.3645, 0.2441 |
| Water activity | 0.47 ± 0.06 | 0.41 ± 0.01 | 1.6617, 0.1719 |
| Moisture content (%) | 5.23 ± 1.85 | 5.73 ± 0.35 | 0.3171, 0.2641 |
| Outlet temperature | 87.25 ± 3.25 | 84.46 ± 4.25 | 1.3658, 0.2214 |
n=3, significant at p <0.05.
Physic mechanical properties
The optimized SDE exhibited a tap density of0.470 ± 0.017 g/mL, bulk density 0.367 ± 0.012 g/mL, Carr's index 21.99 ± 1.06%, and a flow rate 2.95 ± 0.17 g. s-1 (using an exit orifice of 8 mm).
Particle morphology and size
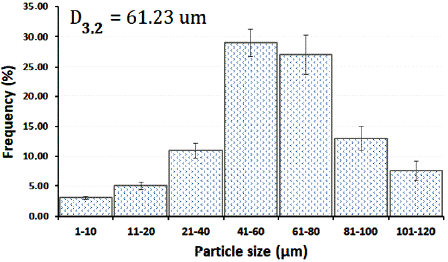
The grains morphology of the optimized SDE of Calophyllum brasiliense was evaluated by SEM. Microphotographs obtained at 1000 (figure 7A) and 5000x (figure 7B) of magnification can be observed the presence of shape-rounded particles of diverse sizes, with clusters of particles of minor sizes. The SDE showed a normal distribution of particle size (figure 8) with a Sauter mean diameter (D3,2) of 61.23 fxm. The data analyses for normality showed standardized skewness of 0.87 and kurtosis of -0.59.
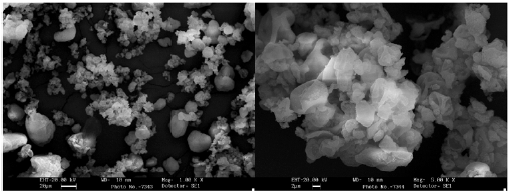
Figure 7 Scanning electronic microphotographs of the optimized spray dry extract of Calophyllum brasiliense Cambés.
Flavonoids and gallic acid content
The spray dried extract of Calophyllum brasiliense Cambés content 9.5 ± 0.52% of gallic acid and 4.85 ± 0.81% of flavonoids as quercetin.
Antioxidant activity
The antioxidant effect of the SDE and the capacity to inhibit a-glycosidase and pancreatic lipase is presented in table 7. The blank solution showed neither antioxidant activity nor a-glycosidase and pancreatic lipase inhibitory effect. The antioxidant effect of the SDE (IC50 1.83 ± 0.90 μg/mL) was not statistically different from the gallic acid (IC50 2.87 ± 0.95), the standard antioxidant compound (t=148.56; p=0.1478).
Table 7 Antioxidant effect and enzymatic inhibition capacity of the spray dried extract.
| Substance | IC5o (μg/mL) | ||
|---|---|---|---|
| DPPH | a-glicosidase | Lipase | |
| Blank | Ф | Ф | Ф |
| Dry extract | 1.83 ± 0.90 a | 74.45 ± 1.45 a | 27.33 ± 2.70 a |
| Gallic acid* | 2.87 ± 0.95 a | - | - |
| Acarbose** | - | 65.30 ± 0.60 b | - |
| Orlistat** | - | - | 0.29 ± 0.01 b |
Control. Ф: do not inhibit. Different letters in a column denote statistically significant differences.
Lipase and a-glycosidase inhibition
The t-test showed a statistically significant difference (t=6.24; p=0.0234) between the inhibitory effect of a-glycosidase produced by SDE (IC50 74.45 ± 1.45) and Acarbose* (IC50 65.30 ± 0.60) (table 7). On the other side, SDE exhibited a lipase inhibitor effect (IC50 27.33 μg/mL) less potent and statistically different (t=15.15; p=0.0001) of Orlistat® (IC50 0.29 μg/mL).
Antiglycation effect
The potential of SDE to inhibit the end-glycation products (AGEs) formation by both the oxidative and the non-oxidative pathway was evaluated (table 8) using bovine serum albumin (BSA) as model protein. By the oxidative way, SDE showed an IC50 of 9.85 μg/mL, while by the non-oxidative pathway exhibited an IC50 of 27.55 μg/mL. In both cases, the effect of the SDE was stronger than the controls (quercetin by the oxidative pathway and aminoguanidine by the non-oxidative one).
Table 8 Antiglycation effect of the spray dried extract of Calophyllum brasiliense Cambés.
| Test Substance | Oxidative Pathway | Non-Oxidative Pathway | ||
|---|---|---|---|---|
| Inhibition (%) (at 100 μg/mL) | IC50 (μg/mL) | Inhibition (%) (at 100 μg/mL) | IC50 (μg/mL) | |
| Spray dried extract | 99.25 ± 3.25 a | 9.85 ± 1.25 a | 98.55± 5.78 a | 20.77± 3.27 a |
| Quercetin | 86.55 ± 4.18 b | 36.25 ± 2.92 b | - | - |
| Aminoguanidine | - | - | 80.75 ± 6.24 b | 27.35 ± 4.71 b |
Different letter in the column indicates statistically significant differences at p <0.05.
Hypoglycemic activity
The blood glucose level of animals of the group I kept between 95-105 mg/dL (figure 9) during the experiment, however, in diabetic animals without treatment (group II) it increased to 335 mg/dL. The blood glucose of the animals treated with glibenclamide 25 mg/kg (group III) was reduced to normal levels in 10 days. All groups treated with the SDE (groups IV to VII) reduced the glucose levels. SDE at 5 mg/kg (groups IV) and 25 mg/kg (group V) was not sufficient to reduce the blood glucose of animals to normal levels. However, doses of 50 mg/kg (group IV) and 100 mg/kg (group V) reduced blood glucose to normal levels in 15 days. No significant statistical difference was observed between the effect of glibenclamide (25 mg/kg) and the SDE at 50 and 100 mg/kg (p <0.05).
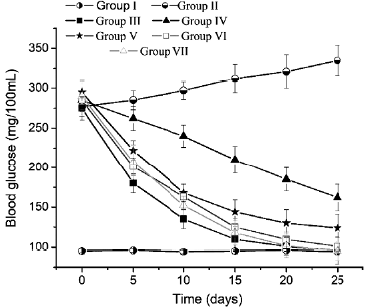
Figure 9 Hypoglycemic effect of the spray dry extract of Calophyllum brasiliense Cambés in Alloxan induced-diabetic rats. Group: I, healthy group, treated with one mL of distilled water; II, diabetic group, received one mL of distilled water; III, diabetic group, received daily, glibenclamide 25 mg/ kg; groups IV-VII, diabetic groups treated daily with 5, 25, 50 and 100 mg/kg of SDE, respectively.
Throughout the experiment, the animals of all groups drank water and ingested food normally, except the animal of the group II, which reduced the food consumption and increased the intake of water (table 9). The animals' body weight of all groups, at the day 0, did not present statistically significant differences, however, the day 25, animals of the group II (diabetics without treatment) exhibited bodyweight higher and statistically different than the rest of the groups (p <0.05).
Table 9 Intake of food and water, body weight, and total serum cholesterol and triglycerides of experimental groups during the evaluation of the hypoglycemic effect of the spray dried extract of Calophyllum brasiliense.
| Parameter | Day | Group I | Group II | Group III | Group IV | GroupV | Group VI | Group VII |
|---|---|---|---|---|---|---|---|---|
| Water intake | 25 | 32.55 ± 4.56. | 38.25 ± 3.27b | 30.45 ± 2.58' | 33.25 ±4.10' | 32.58 ± 3.91' | 32.72 ± 5.16• | 31.29 ± 4.22. |
| Food intake (g) | 23.50 ± 2.35' | 15.33 ± 3.37b | 24.11 ± 4.63. | 23.42 ± 3.33. | 24.58 ± 4.60. | 23.77 ± 3.19' | 25.88 ± 3.01' | |
| o | 155.05 ± 6.51' | 158.55±6.55' | 157.21 ± 4.99. | 159.28 ± 7.39. | 159.81± 4.70' | 156.99 ± 6.19' | 158.77 ± 7.21' | |
| Bodyweight (g) | 15 | 167.33 ± 7.58' | 178.82 ± 5.11• | 169.96 ± 6.01' | 16s.2s ± s.1o· | 167.45 ± 6.60• | 167.97 ± 5.31' | 165.55 ± 4.55. |
| 25 | 175.35 ± 5.11' | 192.40 ± 6.88b | 177.25 ± 8.22. | 175.85 ± 4.69. | 176.83 ± 4.09' | 174.22± 6.70' | 1n.1o ± s.ss· | |
| o | 91.33 ± 2.25 | 138.45 ± 3.36• | 138.91 ± 3.82. | 142.44±4.55' | 142.48 ± 4.02. | 141.37 ± 2.74' | 144.70 ± 3.77' | |
| Cholesterol (mg/dL) | 15 | 92.57 ± 2.85 | 148.41 ±5.66• | 145.86 ± 4.02. | 139.41 ± 2.32. | 136.47 ± 3.33' | 133.66 ± 3.28' | 102.06±4.20' |
| 25 | 90.21 ± 2.16• | 162.35 ± 4.80b | 152.83 ± 3.05. | 131.53 ± 4.17' | 128.55 ± 3.57d | 128.41 ±5.11' | 92.00 ± 4.01. | |
| Triglycerides (mg/dL) | o | 52.25 ± 1.26. | 192.12 ± 3.00' | 193.25 ± 2.25' | 195.12 ± 3.17' | 185.08 ± 4.27• | 196.32 ± 3.37' | 192.02 ± 3.19' |
| 15 | 53.15 ± 1.26• | 214.11 ±3.17' | 209.33 ± 3.55' | 164.02 ± 2.87' | 130.22 ± 4.27• | 116.32 ± 3.01' | 74.00 ± 2.08' | |
| 25 | 51.37 ± 2.09' | 244.1S± 4.99b | 249.35 ± 3.57' | 111.28 ±4.18d | 99.82 ± 3.93. | 65.49 ± 3.39' | 52.30 ± 3.01' |
Group: I: healthy group, treated with one mL of distilled water; II: diabetic group, received one mL of distilled water; III: diabetic group, received daily, glibenclamide 25 mg/kg; groups IV-VII:diabetic groups treated daily with 1, 10, 100 and 1000 mg/kg of SDE, respectively. Normal values: serum total cholesterol 80-130 mg/dL; serum triglycerides 43-110 mg/dL [20].
During the experiment, the total serum cholesterol of the group I kept between 91 and 93 mg/dL, differently from group II where increased to 162.35 mg/dL at the end of the experiment. (table 9). In the group III the serum cholesterol increased to 152.83 mg/dL, the day 25. In groups IV and V the serum cholesterol increased slightly above of the superior normal limit of 130 mg/dL [20], however, group VI and group VII showed a strong diminution of total serum cholesterol, reaching (the day 25) values statistically equal to the Group I (normal control).
Serum triglyceride of the group I showed normal values along the experiment (51-54 mg/dL). The group II exhibited elevated values of serum triglycerides along the time reaching 244.15 mg/mL, at the day 25. In the group III it was observed an increase of the serum triglycerides up to 249.35 mg/dL (table 9), however, the groups IV to VII diminished the serum triglycerides to normal values after at the day 25 (group IV) and at the day 15 the groups V, VI and VII. Notice that, the day 25, the serum triglyceride of the group I and VII were not statistically different (p >0.05).
DISCUSSION
CBE appears as a brownish liquid, having a strong woody aroma with a bitter and astringent flavor. The content of phenolics (1.27 ± 0.10%, expressed as gallic acid) and flavonoids (0.65± 0.12%, as quercetin) provided the woody aroma and the astringent taste to the extract. The extraction method used here differs from that proposed by Carvalho and coworkers whose made a hot extraction at 45 °C for 96 h, using a drug/ solvent ratio of 1:8 (v/v) [6]. Our methodology is simpler than that, exhausting the drug in just 3 days at room temperature, producing an extract with higher solids content (2.87%).
Throughout the investigation for obtaining pharmaceutical products based on plant extracts is crucial to establish physic-chemical parameters (properties) for controlling the quality and warrantee the product reproducibility. The content ofphenolics, flavonoids, total solids, and other properties like density (0.787 g/mL, at 25 °C), refractive index (1.543), and pH (5.49) could be used, preliminarily, for controlling the quality of CBE. Up to our knowledge, there are no technological studies about the preparation and physic-chemical characterization of the extract of Calophyllum brasiliense Cambés.
Botanical extracts submitted to spray drying processes, generally produce sticky materials that adhere to the wall chamber, diminishing the product recovery, hampering the yield, and having unacceptable technological properties [21]. To avoid these problems, solid excipients known as drying aids (adjuvants) are using. An appropriate selection of the drying aids is crucial for obtaining vegetal dried extracts with suitable physic-mechanical properties [22]. In this work, the effect of different combinations of drying aids in the dried extract properties was evaluated, for selecting those providing the high recovery of the dried material (yield), the high content of phenolics, and the minor moisture content (table 3).
The higher phenolic content, and higher yield as well as the minor moisture content, was obtained using lactose monohydrate (5.0%) and colloidal silicon dioxide (0.25%). The drying with maltodextrin or microcrystalline cellulose, instead lactose, had minors yield and produced extracts with higher moisture content. This result contradicts diverse works where the use of microcrystalline cellulose as drying aids for vegetal extracts produced excellent materials. However, there is not a rule, and the drying adjuvants need to be evaluated on a case-by-case basis.
A drying method is as good as its capability to preserve the drug. The critical response in the CBE drying process is the phenolic content. The amount of phenolics in the SDE is a measure of preservation of the actives during the drying process. The antioxidant, hepatoprotective, and hypoglycemic effect of CBE are associated with phenolics and flavonoids content. The inlet air temperature was the factor that most affected the phenolics content. This response was negatively correlated with the air inlet temperature. On the contrary, Phenolics content was positively correlated with the feed flow rate and was practically no affected by the air-drying speed. The higher amount of suspension in the drying chamber, due to the higher feed rate, increases the amount of heat interchanged, diminishing the chamber temperature avoiding the phenolics' degradation. This result agrees with Kha and coworkers [23], which observed that temperatures above 110 °C produced a significant loss of phenolics with consequent loss of the antioxidant activity. The fitted model for phenolics (quadratic model) showed excellent agreement (difference of 0.0349) between the goodness of fit (R2) and goodness of prediction (Q2). Difference minor than 0.3 between these values suggest good model predictability.
In spray drying processes, the product recovery - yield - depends not only on the drying parameters but on the characteristics of the product to be dry and the use of drying aids, among other factors. Usually, the drying of plant extract produces sticky materials and low processes yield. Drying aids are used for obtaining a high product recovery, free-flowing materials, and for improving the extract solubility, and stability. The feed flow rate of the suspension was the factor that most affected the yield. The yield was inversely correlated with inlet air temperature and the air-drying speed. This result could be explained because of the higher the inlet air temperature, the higher the heat transfer with a high loss of heat to the environment. On the other side, the increase in feed flow rate could result in a higher yield, probably, due to a decrease in the average drying temperature [24]. In both cases, the product recovery increases, improving the yielding. The yield data fitted a reduced cubic model showing high values of R2 and Q2 and a difference of 0.0914 between these values, suggesting good model predictability [15].
Water activity and the moisture content are critical properties of pharmaceutical products. Low moisture content and low water activity are necessary for obtaining suitable physic-mechanical properties [25]. Water activity below 0.60 impairs microbial proliferation improving product stability [26]. The water activity and moisture content are associated and depend on the evaporation rate of liquid from the particle, which is governed by the quantity of heat interchanged during the process [16, 25], among other thermodynamical factors. The moisture content of SDE in all runs kept below 7.29%, and the water activity remained below 0.62. These values are suitable for maintaining the microbiological stability of solid pharmaceutical products. As was expected, the higher the inlet air temperature and air-drying speed, the lower the moisture content and water activity due to the increase of the water evaporation rate and the decrease of the resident time of the extract in the drying chamber [21, 27]. In the same way, the combined effect of feed flow rate and air-drying speed reduce the moisture content and water activity (coefficient with a negative sign, table 5).
The outlet temperature depends on factors like the inlet air temperature, and the heat transferred from the drying gas to the solid material during the evaporation process [27]. In this work, the outlet air temperature was inversely correlated to feed flow rate and the air-drying speed (figure 5A, table 5). This result agrees with Quek et al.[28], which reported that the increase of feed flow rate diminished the outlet air temperature and raised the amount of material adhered to the wall chamber. The outlet air temperature fitted a linear model with high values of R2 and Q2 (0.9525 and 0.9416, respectively). Figure 5B exhibits the linear response surface of these responses due to the positive effect of the factors (table 5). The higher the outlet temperature, the minor the moisture content, and water activity [27]. However, with the increase in outlet air temperature, a diminution of the phenolic content was observed. This result agrees with the work of Cortés and coworkers during the production of the spray-dried extract of B. pilosa [2], which informed the same behavior for outlet temperature.
The process optimization ensures the reproducibility of drying processes and the obtainment of dried materials of high quality. The responses used for optimization were phenolics content, yield, outlet temperature, and the water activity, due to the high predictability of the fitted models [15]. The optimization aimed to establish processing parameters (values of factors), for producing an SDE with a high content of pheno-lics, high yielding, and low moisture and water activity. The desirability function had a value of 0.731. The closer to one the desirability value the higher probability to achieve the desired goals [26]. All the responses showed no statistical differences between the observed and predicted values (table 6). This result allows validating the spry-dryer performance using the operational conditions predicted by the optimization tool.
The physic mechanical properties and particle shape and size of the optimized SDE were evaluated. Particle shape and particle size are properties to be considered in solid pharmaceutical materials. SDE exhibits rounded particles (figure 7A) with a Sauter mean diameter of 61.23 cm. The particle size distribution showed normality with kurtosis and skewness between ± 2 [15]. Particles of minor sizes probably formed due to a low number of drying aids in their formation were observed. The trend to form clusters (figure 7B) could result from a high viscosity and surface tension of the liquid suspension, and probably, by a faster grain consolidation [27]. The rounded particles presents in SDE, with a normal size distribution, and a low moisture content of SDE could explain the good flowability (2.95 g/s; orifice 8 mm) and compressibility (Carr' index 21.99%) of this product [15]. Size distribution, particle shape, and moisture are crucial properties for storing and handling solid materials, especially during the implementation of pharmaceutical operations as mixing and compression [15, 29]. The optimization of the drying process and the use of drying aids allows obtaining an SDE with suitable physic-mechanical properties for use in solid forms, like granules, capsules, and tablets.
The use of botanical extracts is as an excellent way for treating multicausal disease like diabetes. Besides the multiple mechanisms acting together, plant extracts produce few adverse effects and are well-accepted by patients. One of the mains uses of the extract of CBC is for reducing blood glucose of diabetic patients [30, 31]. However, are lacking studies evaluating possible hypoglycemic mechanisms of CBC extracts. In this context, the antioxidant activity of SDE and its capability to inhibit a-glycosidase were evaluated. In both assays, the Blank solution does not show inhibitory activity, indicating that the effect produced by the SDE is produced by compounds present on it. SDE showed an excellent antioxidant effect, that was not statistically different from the gallic acid (t=1.56; p=0.1478, at p <0.05). In the same way, SDE inhibited αα-glycosidase at IC50 of 74.45 μg/mL. The effect was slightly minor and statistically different than Acarbose® (IC50= 65.30 μg/mL). Phenols and flavonoids are OH-rich compounds that could be bonding to carbohydrates impairing the normal enzymatic function. On the other side, they are responsible for the strong scavenging of free radicals, producing a potent antioxidant effect [7]. Alpha-glycosidases hydrolyze complex carbohydrates for forming simple carbohydrates [31]. The inhibition of a-glycosidase avoids the sharp increase of the postprandial carbohydrates level in blood, improving the glycemic control [31]. SDE also inhibited pancreatic lipase showing an IC50 of 67.49 μg/mL but was less potent than Orlistat® (IC50 0.29 μg/mL). In obese diabetics, the use of lipase inhibitors could be an effective way of lowering fat absorption, reducing the risks associated with type II diabetes. Drugs a-glycosidase and pancreatic lipase inhibitors are scarce. Botanical extracts with both effects are still more reduced remarking the potential of this extract as a therapeutic agent.
The SDE showed a potent antiglycation effect with IC50 9.85 μg/mL by the oxidative pathway and 20.77 μg/mL by the non-oxidative one. By both pathways, the effect was more potent than the control quercetin 36.25 μg/mL (oxidative way), and amino-guanidine 27.35 μg/mL (non-oxidative way). Blood carbohydrates are augmented in uncompensated diabetics. Advanced glycation end products (AGEs) are produced by the reaction of lipids and/or proteins with reduced sugars. They are the main cause of degenerative diseases associated to diabetes like atherosclerosis, chronic kidney disease, and retinopathy [32]. Although the antiglycation effect of plants extract has not been established, some studies suggest that may act hindering the formation of AGEs by preventing further oxidation of Amadori products [33]. This effect can be potentiated by the strong antioxidant effect produced by the SDE, in which phenols and flavonoids plays a special role. In this sense, must be take on account the strong antiglycation effect of the SDE by the oxidative pathway with IC50 9.85 μg/mL.
In this work, the hypoglycemic effect of SDE in diabetic rats was evaluated using four doses (5, 25, 50, and 100 mg/kg of body weight). Animals of all groups consumed food and drank water normally, except in the group II, which consumed a smaller amount of food that was statistically different from the rest of the groups. This probably happened by the uncontrolled increase of carbohydrates in blood as the animals get worse because they were not treated. The body weight increased in all groups, however, in the group II was higher and statistically difference from the rest of the groups.
Low doses of the SDE (5, and 25 mg/kg) were not effective for reducing the blood glucose of the diabetic rats (groups IV and V), however, doses of 50 and 100 mg/ kg produced a potent blood sugar reduction, that was not statistically different from the effect of Glibenclamide 25 mg/kg (group III), onward the day 15 of the experiment. The potent hypoglycemic effect of the SDE could be occurs via inhibition of a-glycosidase, and due to the strong antioxidant effect of this extract.
The SDE lowered total serum cholesterol and triglyceride levels of diabetic animals, being the effect more potent at 100 mg/kg (group VII). The reduction of carbohydrates blood levels onward the day 15 allow improves cholesterol and triglycerides levels. This result agrees with that reported by Carvalho and co-workers who prepared granules for using a liquid extract of CBC [6]. On the other side, the antioxidant effect of the SDE and the inhibition of pancreatic lipase produced by this extract can actively contribute for reducing serum triglycerides.
The spray dried extract of CBC produced a potent hypoglycemic effect in diabetics animal, however, despite the animals has consumed a normal food, after the day 15 the blood glucose level, total serum cholesterol and triglyceride were normal, allowing that the animals maintaining a normal live eating food and drinking water normally. These results must be associated to the effect of the SDE, which could have controlled the diabetes symptoms by three different mechanisms: via antioxidant, via a-glycosidase and lipase inhibition, and via antiglycation. SDE of Calophyllum brasiliense Cambés arise as a promissory standardized product, which could be used properly in diabetic/ obese patients.
CONCLUSIONS
There are evidence suggesting the use of plant products as an excellent therapeutic strategy for treating multicausal diseases like diabetes. The diversity of compounds presents in the extracts produces a multi-target effect synergizing the beneficial response of patients. The SDE optimized and characterized in this work exhibited excellent hypoglycemic effect in vivo, probably mediated by the potent antioxidant and antiglycation activity, and the powerful capability for inhibiting a-glycosidase. At the same time, SDE at 100 mg/kg reduces the serum cholesterol and triglycerides, probably mediated by the inhibition of pancreatic lipase. The SDE of Calophyllum brasiliense Cambés arise as a promissory hypoglycemic product due to the capacity to activate different mechanisms associated to diabetes control. However, other studies must be done for evaluating the real utility of SDE in diabetics.