INTRODUCTION
The enterobacteriales comprises a large group of Gram-negative, glucose-fermenters and non-spore-forming bacteria with typically 0.8-5.0 μm in length. These microorganisms are widely distributed, been founded in various sites of the human and animal body, as well as in the environment and food samples [1]. Although strains of some species are harmless commensals, others are potentially pathogenic to humans, animals, insets and plants. The members of this order are closely related with the most common opportunistic infections and are associated to several complex diseases including septicemia, pneumonia, meningitis and urinary tract infections [2].
Generally, for the treatment of infections caused by enterobacteriales, several antimicrobials are used, especially the class of beta-lactam antibiotics (e.g., penicillins, cephalosporins, monobactams and carbapenems) [3,4]. In susceptible microorganisms, the beta-lactam antibiotics are bactericidal agents that irreversibly inhibit the enzyme transpeptidase that catalyzes reactions between the peptideoglycan chains of the bacterial cell wall [5]. Among these antimicrobials, ampicillin, a semi-synthetic penicillin discovered in 1961, has an important role in therapy of enterobacteriales infections. However, as with other antimicrobials, the exacerbated use of ampicillin as prophylactic or therapeutic in veterinary and agrarian practices contributes to emergence and dissemination of resistance against this compound [6].
It has been described that the main mechanism of resistance to ampicillin in enterobacteriales is the production of Extended-spectrum β-lactamases (ESBLs) [7]. ESBLs are a rapidly evolving group of β-lactamases which share the ability to hydrolyze penicillins; first-, second-, and third-generation cephalosporins; and aztreonam (but not the cephamycins such as cefoxitin or carbapenems), and are inhibited by β-lactamase inhibitors such as clavulanic acid [8-10]. The majority of ESBLs are derived from the widespread broad-spectrum β-lactamases TEM and SHV. There are also others families of ESBLs, including the CTX-M and OXA-type enzymes as well others unrelated β-lactamases (e.g., PER-1, PER-2, VEB-1, CME-1, TLA-1, GES-1) [11]. The ESBLs are widely distributed and can spread easily since the genes encoding them can be transferred via mobile genetic elements, such as plasmids, transposons and integrons [12]. In the clinical setting, ESBL-producing enterobacteriales are associated with increased mortality rates and high health costs [13].
The increase in infections caused by ESBL-positive enterobacteriales led to a greater use of carbapenems, thus promoting the emergence of resistant strains to this class [14]. Bacterial resistance to carbapenems is observed due to the reduction of bacterial outer mem-brane permeability, expression of efflux pumps, site mutation and enzymatic hydrolysis (i.e., carbapenemase production) of the antimicrobial; this latter is of greater concern to the biomedical community due to the high potential of dissemination interspecies [15]. The most frequent carbapenemases in Gram-negative bacteria belong to class A (Klebsiella pneumoniae carbapenemase- KPC; Guiana extended spectrum-GES); class B (New Delhi metallo-beta-lactamase-NDM; Verona imipenem-VIM; Imipenem-IPM) and class D (Oxacillinases-OXA-48, OXA-181) from Ambler and are transferred by mobile genetic elements. These enzymes can be detected using phenotypic tests, but detection of the gene encoding bla is considered gold standard [16-18].
Beta-lactamase-producing bacteria outside the clinical setting have showed to contribute with the postulated decline of the efficacy of beta-lactam antibiotics worldwide. Especially raw sewage seems to play a key role in this spread, as they serve both as habitats and as trans-port systems for microorganisms. For instance, in Brazil is estimated that approximately 20 % of the medicaments are discarded in the sewage network or household wastewater [19]. Regarding the antimicrobials, they are not fully metabolized in the body, and are released to the effluent through excreta from the patient. A major concern is the easy adaptation of bacterial population, since the subconcentrations of antimicrobials may exert selective pressure on the bacterial, selecting resistant strains [20]. As a result, these environments have been considered reservoirs of bacterial resistance to antimicrobials [21].
However, contrary to clinical settings, where the distribution of resistant bacteria is well documented, distribution and evidence of resistant pathogens in the domestic sewage has been little explored, especially in developing countries such as Brazil. Our group already has showed that Pseudomonas aeruginosa isolates from a domestic wastewater treatment plant (WWTP) localized in Minas Gerais state (Brazil) is able to carried beta-lactamases (AmpC) and has considerable resistance rate to carbapenems (11 %), suggesting that the sewage is a potential reservoir of resistance determinants against beta-lactam in this region [22]. Thus, considering the importance of beta-lactam against glucose-fermenter Gram-negative bacilli and the circulation of ESBL and carbapenemase-producing microorganisms in sewage, this study aimed to investigate the occurrence of ESBL and carbapenemases between ampicillin-resistant enterobacteriales recovered from a municipal raw sewage in Minas Gerais, Brazil.
MATERIAL AND METHODS
Sample collection
One liter of sewage in direct and spontaneous drainage for a watercourse in the city of Divinópolis (MG) (geographical coordinates: 20° 08 '20 "S and 44° 53' 02" W), localized in southeast Brazil (232 945 inhabitants), was collected on 6th of June 2017 [22]. The sample was placed in pre-sterilized screw-cap polypropylene vial, stored on ice until analyzed and was processed within two hours of collection at the Laboratório de Diganóstico Laboratorial e Microbiologia Clínica at the Universidade Federal de São João del-Rei (Divinópolis-MG/Brazil).
Cultures and bacterial isolation
In order to isolate the enterobacteriales species, the entire sewage sample was centrifuged and then 2 grams of the sediment was inoculated into BHI broth (Isofar, Brazil) plus ampicillin (640 μg/mL) (Sigma-Aldrich, Germany). Subsequently, a serial dilution of the 100 μL sample of each dilution and of the raw sample was inoculated on MacConkey agar (Isofar, Brazil) added of ampicillin (288 μg/mL) (Sigma-Aldrich, Germany). The concentration of ampicillin used is in accordance with the Manual of the Clinical Laboratory Standards Institute M100 S28 where enterobacteriales isolates for which the minimum inhibitory concentration (MIC) is ≥ 32 μg/mL are considered resistant to this antimicrobial [23].
In order to identify the bacterial species, the Gram staining technique and biochemical-physiological tests were used [24], including the production of oxidase enzyme, fermentation of carbohydrates (glucose, lactose and sucrose), pigment production, lysine decarboxylation, indole production, H2S production, use of citrate and malonate as the carbon source and urease production. In addition, chromogenic agar (Renylab, Brazil) was also used to identify the colonies, according to the manufacturer's instructions. Subsequently, the bacterial isolates were inoculated in nutrient agar to verify the purity of the culture by evaluating the morphology of the colonies. From this culture, three to five colonies were suspended in BHI broth and incubated at 37 °C for a period of 18-24 h. After this incubation, 500 μl of the culture was added with 25 % v/v glycerol and stored at -80 °C.
Beta-lactams susceptibility test
The antimicrobial susceptibility profile of aztreonan (ATM), ceftazidime (CAZ), cefotaxime (CTX), ceftriaxone (CRO), amoxicillin with clavulanic acid (AMC) (Sensifar) and carbapenens (meropenem, MEM; imipenem, IMP and ertapenem, ERT) (Cecon, Brazil) was determined by the standard agar diffusion technique and interpreted according to the Manual of the Clinical Laboratory Standards Institute M100 S28, considering the breakpoints established by it. Escherichia coli ATCC 25 922 was used as control of the experiments [23].
Phenotypic detection of the production of carbapenemases and ESBL
The enterobacteriales isolates were submitted to phenotypic tests to determine the production of carbapenemases using the modified Hodge test (MHT) and inactivation of carbapenems (mCIM and eCIM). Briefly, the MHT was performed using an E. coli ATCC 25 922 inoculum prepared according to the 0.5 McFarland standard in nutrient broth (Isofar, Brazil). Subsequently, this suspension was inoculated onto the surface of a Mueller-Hinton agar (Isofar, Brazil) with [25] and without [23] 50 microliters of Triton X-100 previously added. Next, a meropenem disk was placed in the center of the plaque. Using a 10 μL loop, three to five colonies of each test species freshly grown in nutrient agar (Isofar, Brazil) were inoculated in a straight line from the end of the plate to the center of the disc. After incubation at 35 ± 2 °C for 16-20 h, the plate was analyzed. If a carbapenemase is produced by the test bacterium, the antimicrobial will be hydrolyzed, allowing E. coli ATCC 25 922 to grow toward the disc, creating a distortion of the inhibition halo.
To perform the mCIM test [23], in turn, three to five colonies of each bacterial iso-late grown from blood agar were inoculated into 2 mL of nutrient broth (Isofar, Brazil). Simultaneously, a meropenem disk was placed in each test tube and it was incubated at 35 ± 2 °C for 4 h. Upon completion of the incubation, a suspension of E. coli ATCC 25922 with turbidity pattern compatible with the 0.5 McFarland scale was inoculated on the Mueller Hinton agar plate (Isofar, Brazil). The meropenem disks removed from the tubes were placed on the Muller Hinton agar plate previously inoculated with the E. coli ATCC 25922 indicator strains and the plates were incubated at 35 ± 2 °C. If carbapenemase is produced, the carbapenem is inactivated during incubation, allowing the growth of E. coli ATCC 25922, with the formation of an inhibition halo of less than 15 mm [23]. Simultaneously with the mCIM test, the eCIM test [23] was performed, which presents as a modification of the procedure described above, the use of a tube of nutrient broth from each isolate containing 20 μL of 0.5 M EDTA. According to CLSI [23], this procedure allows the differentiation of carbapenemases type. In experiments to search of carbapenemase, E. coli ATCC 25 922 and clinical carbapenemase-positive- K. pneumoniae were used, respectively, as negative and positive controls.
In addition, the isolates were submitted to the phenotypic test to determine ESBL production, using the antimicrobial substrates CAZ, ATM, CRO and CTX, according to CLSI protocol [23]. Briefly, a suspension of each bacterial isolate (concentration 108 UFC/mL) was inoculated into Muller-Hinton agar (Alere, USA). The antimicrobial discs were then arranged around the AMC disc at a distance of20 mm and incubated at 37 °C for 24 h. The isolate is considered an ESBL producer when a halo distortion of antimicrobial substrate is observed after incubation. E. coli ATCC 25922 and clinical ESBL-positive- Klebsiella pneumoniae were used, respectively, as negative and positive controls.
RESULTS AND DISCUSSION
Currently, raw sewage is recognized as one of the most important routes for propagation of AMR genes worldwide. Recent evidences showed that antibiotic resistance in urban sewage mirrors the pattern of clinical antibiotic resistance prevalence, making the search for resistance determinants in these residues an indirect way of epidemiological surveillance of multi-resistant microorganisms [7]. Despite the large number of studies that highlights the importance of sewage in dissemination of antibiotic resistance in Europe and EUA, the dynamic of this phenomenon has been little explored in Brazil [26]. However, Hendriksen et al. [ 27], in an metagenomic analysis of untreated sewage to characterize the bacterial resistome from 79 sites in 60 countries, showed that the Brazil had the highest abundance of AMR genes of all countries studded (4616.9 fragments per kilobase per million) (Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage). Thus, we have aimed to determine the resistance profile and presence of beta-lactamase among ampicillin-resistance enterobacteriales recovered from an urban raw sewage in a medium-sized city from southeast Brazil.
Here, 45 bacterial isolates were recovered from the domestic sewage in ampicillin-enriched medium (640 μg/mL). Overall, five different species were identified, been them Escherichia coli (37/45; 82.2 %), Klebsiella pneumoniae (4/45; 8.9 %), Klebsiella oxytoca (1/45; 2.2 %), Citrobacter freundii (1/45; 2.2 %) and Pantoea agglomerans (1/45; 2.2 %). In fact, these species are often recovered in both domestic [28,29] and hospital sewage [7], the latter characterized by the great exposure of the bacterial population to various antimicrobials, including ampicillin.
The highest number of E. coli isolates (82.2 %) is possibly associated with the fact that this species is part of the intestinal microbiota of humans and animals. As in our study, this species was also the most recovered by Korzeniewska and Harnisz [30] in a municipal sewage. However, it is worth mentioning that E. coli is also responsible for approximately 50 % of health care-related infections and 70-90 % of urinary tract infections [31]. In fact, acute community-acquired urinary tract infections (CAUTIs) caused by E. coli are the most common bacterial infections worldwide, affecting approximately 150 million persons per year, particularly women [32]. Of particular concern is that the isolates recovered here are resistant to ampicillin, an antimicrobial widely used in the clinical setting.
Species of the genus Klebsiella and Citrobacter were also recovered, but to a lesser extent. These bacteria are considered as coliforms because they inhabit the gastrointestinal tract, but can also be found in pastures, soils and submerged plants [33]. Already the P. agglomerans, formerly known as Enterobacter agglomerans, is a clinically important species. It is associated with plants, but has been the cause of opportunistic infections mainly in plant-related wounds or infections related to health care in immunocompromised patients [34]. Possibly this finding is because this species is present in plants located near the domestic sewage.
All bacterial isolates from this study were submitted to the susceptibility test for several beta-lactam agents by the agar diffusion method (table 1). In general, a moderate beta-lactam resistance rate (14/45, 31.1 %) was observed among the isolates. An important finding in this work was that all the isolates of Klebsiella spp. and C. freundii were sensitive to the tested beta-lactam antibiotics (AMC, CTX, CRO, CAZ, ATM, MEM, IMP, ERT). Thus, our data show that these species may have limited resistance mechanisms, which cover only ampicillin as intrinsic resistance in Klebsiella spp. Corroborating with this study, Klebsiella spp. and C. freundii exhibiting resistance to this beta-lactam have been found in water samples from Latin America. Oliveira [35] relates that 52 % of enterobacteriales isolates from water samples in Porto Alegre-RS (including K. pneumoniae and C. Jreundii) are resistant to ampicillin. In turn, Castañeda et al. [ 36] demonstrated resistance to ampicillin involving all isolates of Klebsiella and Citrobacter recovered from marine water and beach sediments in Venezuela. Possibly ampicillin resistance in these bacteria may be related to the widespread use of this drug, which is available for a longer time in the market.
Table 1 Susceptibility profile to beta-lactam antimicrobials and distribution of ESBL-producing isolates among ampicillin-resistant enterobacteriales recovered from raw sewage.
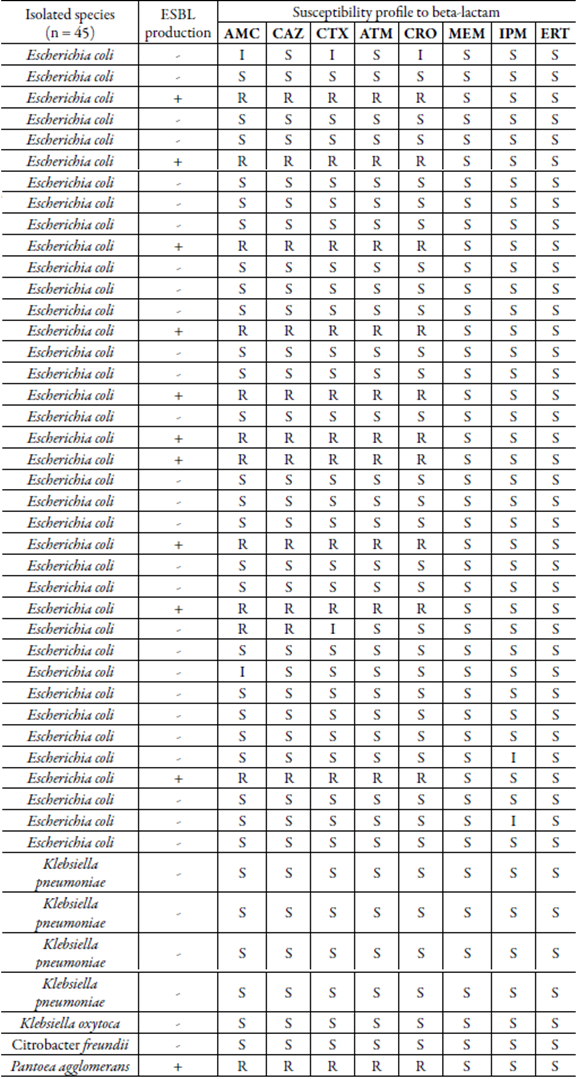
ESBL: Extended-spectrum beta-lactamase; S: Sensible, R: Resistant; I: Intermediary; AMC: Amoxicillin with clavulanic acid; CAZ: Ceftazidime; CTX: Cefotaxime; ATM: Aztreonan; CRO: Ceftriaxone; MEM: Meropenem; IMP: Imipenem; ERT: Ertapenem.
On the other hand, P. agglomerans presented sensitivity only to carbapenems (MEM, IPM, and ERT). Ruiz [37] and Borges et al. [ 38] also showed a high resistance profile in P. agglomerans isolated from hospital sewage and clinical, respectively. In contrast to our finding, Filho [39] related high rate of resistance to carbapenems in this species, including the ertapenem, imipenem and meropenem. Considering the clinical importance of P. agglomerans, studies on the susceptibility profile in this species is relevant and may help in choosing the best antimicrobial therapy.
Nevertheless, resistant E. coli to at least two beta-lactams (11/37; 29.7 %) and with decreased or intermediate susceptibility to amoxicillin/clavulanic acid (2/37; 5.4 %), cefotaxime (2/37; 5.4 %), ceftriaxone (1/37; 2.7 %) and imipenem (2/37; 2.7 %) were found. Carbapenems are the last generation of beta-lactam antimicrobials used to treat infections caused by Gram-negative bacteria resistant to penicillins, cephalosporins and other compounds of the same class [40]. According to Bessa et al. [ 41] high rates of E. coli resistant to different antimicrobials, among them imipenem, are not common in environments such as rivers, domestic sewage and sewage treatment plants. Thus, ours finding of decreased susceptibility imipenem among E. coli isolates from domestic sewage is of particular concern considering the possibility of exchange of bacteria between environmental and clinical settings, which may contribute to the spread and increase of carbapenem resistance [42].
In this context, resistance to carbapenems in enterobacteriales occurs mainly by hydrolytic action promoted by carbapenemases [15], and phenotypic tests have been widely used for detection these enzymes. It should be emphasized that the investigation of the production of carbapenemases is of great importance, since even in isolates considered sensitive to carbapenems, it is possible to harbor the genes coding for this enzyme [43]. Here, using the modified Hodge test (MHT) and inactivation of carbapenems mCIM and eCIM methods, none isolated was carbapenemase-positive. It may be suggested that there is a low prevalence of microorganisms producing carbapenemases in domestic sewage environments in region studded. In fact, Conte et al. [ 44] did not detect the bla gene in E. coli, K. pneumoniae and K. oxytoca recovered from hospital sewage and domestic or industrial sanitary effluent from Curitiba-PR. However, the finding of E. coli with decreased susceptibility to imipenem alerts to the possibility of the presence of non-enzymatic mechanisms of resistance to carbapenems circulating in the investigated environment, such as overexpression of efflux pumps (e.g., AdeABC), penicillin-binding protein alterations, or loss of outer membrane protein (e.g., CarO) [45].
In contrast to the carbapenemases, the phenotypic assay has revealed considerable prevalence of ESBL-producer isolates, which were mostly identified as E. coli (10/45; 22.2 %) (figure 1). Phenotypic test for ESBL detection has not been validated by CLSI [23] for P. agglomerans. But, here, these species presented a test result that would be compatible with the producer interpretation of this beta-lactamase [46,47]. ESBL-producing strains are capable of hydrolyzing penicillins, broad-spectrum cephalosporins and monobactans [3], which limit considerable the therapeutic options available to, basically, the carbapenens and polymyxins. Korzeniewska & Harnisz [30] found similar results in E. coli isolates from municipal sewage samples, but other ESBL-positive enterobacteriales, including the genera Citrobacter and Klebsiella, were also reported. In addition, according to Mesa et al. [ 48], all E. coli (32) recovered from five domestic sewage samples were ESBL producers. However, it should be considered that the present study was conducted in a single sample of domestic sewage, what could explain the greater diversity of enterobacteriales found in other studies as well as the higher rate of ESBL-positive E. coli detected.
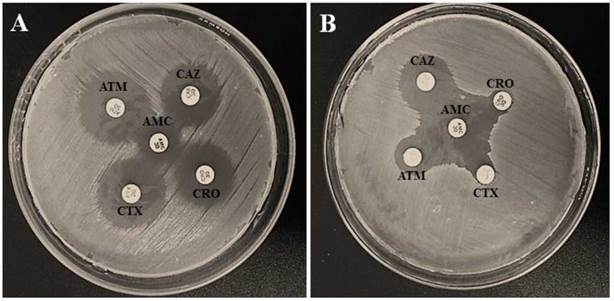
Figure 1 Phenotypic disk approximation test according to CLSI 2017. A) ESBL-Negative test. B) ESBL-positive test. CAZ: Ceftazidime; CTX: Cefotaxime; ATM: Aztreonam; CRO: Ceftriaxone.
Most ampicillin-resistant E. coli were carbapenemase and ESBL-negative and sensitive to the antimicrobials aztreonam, ceftazidime, amoxicillin/clavulanic acid, ceftriaxone, cefotaxime and carbapenens. Thus, possibly mechanisms of resistance to ampicillin other than enzymatic inactivation circulate among the species of the studied environment, including target modification, change in efflux pump and external membrane permeability [5]. In this study, only the production of inactivating enzymes was investigated, and the evaluation of other resistance determinants thought of different phenotypic and genotypic methodologies are needed to elucidate the mechanisms of resistance to ampicillin presented by these isolates.
CONCLUSION
Ampicillin-resistant and ESBL-producing enterobacteriales (mainly E. coli) were recovered from the urban raw sewage and analyzed. Thus, we showed that the domestic sewage is an important source of AMR determinants in medium-sized cities from southeast Brazil. Furthermore, the absence of detection of beta-lactam hydrolytic enzymes (i.e., ESBL and carbapenemases) in many cases, suggests that possibly the mechanism of bacterial resistance to beta-lactams in this environment is non-enzymatic and specific for ampicillin. Despite the ampicillin-resistant, the sensitivity to others beta-lactam antibiotics (e.g., carbapenems) was high, suggesting that these compounds remain as good therapeutic option against microbial population of region. The results showed here not only reinforce the importance of surveillance to improve antibiotic resistance control, but also help to predict the profile of resistance in clinical, since that is well documented the strong relationship between clinical and environmental resistance pattern.














