INTRODUCTION
According to the World Health Organization (WHO), the four main groups of chronic non-communicable diseases (CNCDs), cardiovascular diseases, cancer, chronic respiratory diseases and diabetes are cause of 71 % of global deaths and represent more than 80% of all premature mortality, that is, deaths between 30 and 69 years [1]. Popular medical practices use natural elements in the treatment of a variety of conditions that affect the population, such as chronic non-communicable diseases [2].
Ethnobotanical and ethnopharmacological studies already demonstrated the use of plant species with therapeutic properties by the population [3, 4]. In addition, numerous studies reported the biological activity of different plant species for treat- ing CNCDs, such as Anacardium occidentale L. [5], Artocapus altilis (Parkinson) Fosberg [6], Artocapus heterophyllus Lam. [7], Citrus x aurantium L. [8], Citrus x latifolia Tanaka ex Q. Jiménez [9], Manilkara zapota (L.) Von Royen [10], Persea americana Mill. [11], Psidium guajava L. [12], Spondias mombin L. [13], Talisia esculenta Radlk [14], and Tamarindus indica L. [15].
Plant species produce primary and secondary metabolites, including oxalic acid, which is also present in the form of oxalate salt [16]. Studies have shown that the oxalate content varies in different parts of the plant, but usually the highest concentrations are found in the leaves [17, 18]. The formation of calcium oxalate crystals in the tissues of plant species is related to the accumulation of oxalic acid from the plant metabolism, which later reacts with calcium ions, precipitating in the form of calcium oxalate [19]. According to the diversity of crystals, their spatial distribution and concentration, there are several hypotheses regarding the function of calcium oxalate in plant species [16]. Besides, these oxalate crystals can be arranged in the form of druse [20], prismatic crystal [21], raphide [22] and crystalline sand [23].
The consumption of foods with a high content of calcium oxalate can lead to hyperox-aluria, because once ingested, the calcium oxalate is converted into oxalate, which in the presence of calcium ions present throughout the urinary tract, may precipitate and lead to formation of calcium oxalate crystals, a primary component of kidney stones [24]. According to Duan et al. [25], oxalate induces the autophagy of renal tubular cells, which in turn are related to the formation of kidney stones.
Furthermore, studies warn that the ingestion of fruit juices, such as star fruit, can lead to renal damage due to the high concentration of calcium oxalate [26, 27]. In addition, studies show that chronic non-communicable diseases like metabolic syndrome, diabetes and hypertension are risk factors for the formation of urolithiasis [28 - 30].
Medicinal plants are still considered promising sources of drugs discovery and provide a variety of bioactive chemicals and powerful pharmacological actions [31]. Zeni et al. [32] reported in his study that 85 % of patients use medicinal plants in the form of teas made from the leaves. In ethnobotanical surveys, teas represent the most common method of preparation. The choice of using this method of preparation is probably related to the availability of the plant part used, the leaves, which are available throughout the year [33, 34].
However, despite the widespread use, there are risks in uncontrolled use. Without medical and pharmaceutical guidance or even scientific proof of efficacy and identification, the inappropriate use of some plant species can lead to intoxication, neutralization or potentiation of the pharmacological effect, also leading to aggravation in the clinical condition of the patient, and ultimately, death [35]. Thus, the quantification of oxalic acid contained in plant species can contribute to prevention of possible intoxication, mitigate misunderstandings and their consequences. Therefore, the aim of this study was to quantify the oxalic acid content contained in medicinal plant species and to optimize the quantification methodology present in the literature.
MATERIALS AND METHODS
Species collection and identification
The plant material was collected in the state of Pernambuco, Brazil. Exsiccates were prepared and a voucher specimen was deposited in the Herbarium the herbarium Dárdano de Andrade Lima, from the Agronomic Institute of Pernambuco (IPA): Anacardium occidentale (89 979), Artocapus altilis (91 180), Artocapus heterophyllus (91 181), Citrus x aurantium (89 972), Citrus x latifolia (89 971), Manilkara zapota (90 643), Persea americana (89 997), Psidium guajava (88 150), Spondias mombin (90 666), Talisia esculenta (90 929), and Tamarindus indica (89 998).
Extraction and quantification of oxalic acid
The leaves of each species were dried in an oven for 48 hours straight at 60 °C and then crushed. Subsequently, 0.5 g of the crushed leaves was weighed in erlenmeyer with 100 mL, then added with 50 mL of hydrochloric acid 2 mol.L-1 (Vetec, Brazil) to obtain the acid extract, or 50 ml of distilled water as an extracting solvent to obtain the aqueous extract. After, both extracts were taken to the water bath with magnetic stirring at 80 °C for 30 minutes. The extracts of each species were then filtered and diluted with 50 mL of distilled water [24]. Aliquots with 25 mL of each extract were titrated in triplicate with a potassium permanganate solution 0.02 mol.L-1 (LabSynth, Brazil) at 60 °C and the concentrations of oxalic acid were expressed in g/100g of dry vegetable drug.
Standard curve
The standard curve was built using standard solutions of calcium oxalate (Dinâmica, Brazil) in concentrations of 0.005 mol.L-1, 0.01 mol.L-1, 0.02 mol.L-1, 0.04 mol.L-1 and 0.06 mol.L-1. After a visual linear correlation, the results were analyzed statistically to define the coefficient of determination (R2) and linear equation using the Excel.
Quantification methodology optimization
The two species that obtained the highest oxalic acid content went for optimization. A univariate analysis was performed in which the influence of three parameters was evaluated: extraction time, ranging from 5 to 40 minutes, with a 5 minutes interval; extraction temperature, from 30 °C to 90 °C, varying by 10 °C; and the concentration of the extracting solvent (hydrochloric acid) in concentrations ranging from 0.2 mol.L-1 to 1.8 mol.L-1, with interval of 0.4 mol.L-1. As it is univariate, each stage was incorporated into the result of the previous one.
Precipitation of calcium oxalate for quantification
The optimized extracts of the species were prepared and cooled in the refrigerator for 30 minutes at 10 °C. Then, 15 mL of saturated CaCl2 solution (Dinâmica, Brazil) were added to each extract. The solution containing the precipitate was centrifuged for 10 minutes at 2500 rpm. After centrifugation, the supernatant was discarded, and the precipitates were solubilized in H2SO4 2 mol.L-1. The solution resulting from the solubilization was titrated with potassium permanganate solution 0.02 mol.L-1. Additional precipitate samples of each species were used to check for the presence of post-precipitation calcium oxalate crystals through a polarization microscope (Leica DM750M), attached with a digital camera (Leica ICC50W), through which images were obtained to be processed in software (LAS EZ).
RESULTS AND DISCUSSION
The calibration curve had the equation of the line given by y = 513.58x - 0.2167 where "y" refers to the spent volume of potassium permanganate and "x" the concentration in mol.L-1 of oxalate. The determination coefficient (R2) was 0.9988, proving the adequacy of the method to the evaluated range. The recommendation for (R2) is a minimum value of 0.99 [36].
The results presented in table 1 shows that all species analyzed have a different amount of oxalic acid. The analyzes of average concentration of oxalic acid among the 11 species chosen varied by 291.04 % for the acidic extracts and 356.49 % for the aqueous extracts. Furthermore, the concentrations obtained from oxalic acid were different even in some species that belongs to the same family.
Table 1 Concentration of oxalic acid (g/100 g) of dry vegetable drug in the 11 species analyzed.
| Species | Solvent | |
|---|---|---|
| HCl 2 mol.L-1 | ||
| Anacardiumoccidentale | 8.31 ± 0.09 | 9.97 ± 0.07 |
| Artocarpusaltilis | 9.01 ± 0.00 | 16.79 ± 0.05 |
| Artocarpusheterophyllus | 17.21 ± 0.07 | 17.91 ± 0.08 |
| Citrus x aurantium | 7.12 ± 0.05 | 16.79 ± 0.05 |
| Citrus x latifolia | 13.43 ± 0.09 | 15.92 ± 0.05 |
| Manilkarazapota | 10.34 ± 0.05 | 12.10 ± 0.04 |
| Persea americana | 10.60 ± 0.06 | 11.12 ± 0.10 |
| Psidiumguajava | 13.92 ± 0.13 | 17.48 ± 0.13 |
| Spondiasmombin | 13.43 ± 0.09 | 15.20 ± 0.10 |
| Talisiaesculenta | 3.77 ± 0.06 | 4.58 ± 0.09 |
| Tamarindus indica | 7.30 ± 0.05 | 16.54 ± 0.07 |
Prasad and Shivay [37] commented that oxalic acid is present in plant species in its molecular form (oxalic acid) or in salt form (calcium oxalate) and that the acid's water solubility is about four times higher in comparison with the oxalate form, which is practically insoluble in an aqueous medium. On the other hand, oxalate salts are easily solubilized in acidified medium with HCl or H2SO4.Traditionally, both soluble fractions (oxalic acid) and insoluble oxalates (oxalate crystals) are extracted using acidified solutions [38]. This could explain why the results of oxalic acid concentration from the extraction utilizing acidic solvent expressed greater outcomes in 100 % of plant species (figure 1) in comparison with aqueous extracts, thus, it is characterized as the best solvent when the aim is to quantify the total oxalic acid present in the samples.
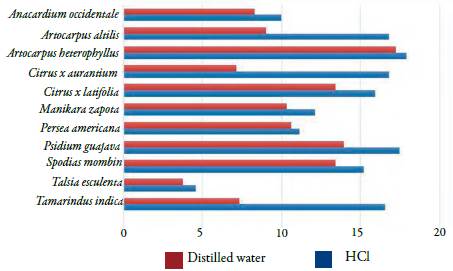
Figure 1 Average concentration of oxalic acid (g/100 g of dry vegetable drug) in different solvents.
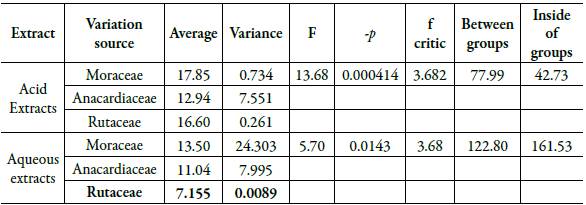
Among plant species that belongs to the same botanical family, the analysis of variance that was performed, for both aqueous and acid extracts, demonstrated a statistically significant variation between the averages obtained from the concentration of oxalic acid in the species Artocarpus heterophyllus and Artocarpus altilis (Moraceae), Spondi-asmombim and Anarcadium occidentale (Anacardeaceae), Citrus x aurantium and Citrus x latifolia (Rutaceae), as it is observed in table 2.
Table 2 Statistical analysis of oxalic acid concentration among botanical families with a significance level of p <0.05.
In the literature, data that show the oxalic acid content in plant species used as adjuvants in the treatment of CNCDs are scarce, however the levels of oxalic acid found in these 11 plant species are higher than the levels found in other studies, which used different species and analysis methodologies. The high results can be explained due to the presence of oxidizable compounds in the plant matrix, since under favorable chemical conditions, potassium permanganate can react with several reducing molecules. Although they may present various stoichiometric proportions of oxidation reduction, parallel reactions caused by the presence of several oxidizable substances can be quantified through determination of the concentration of oxalic acid [39]. Several studies report the oxalic acid content in plant species consumed by the population (table 3). The studies use older techniques, such as titration, even more sophisticated techniques such as high performance liquid chromatography (HPLC).
Table 3 Summarization of studies that quantified oxalate in plant species consumed by the population by different methods.
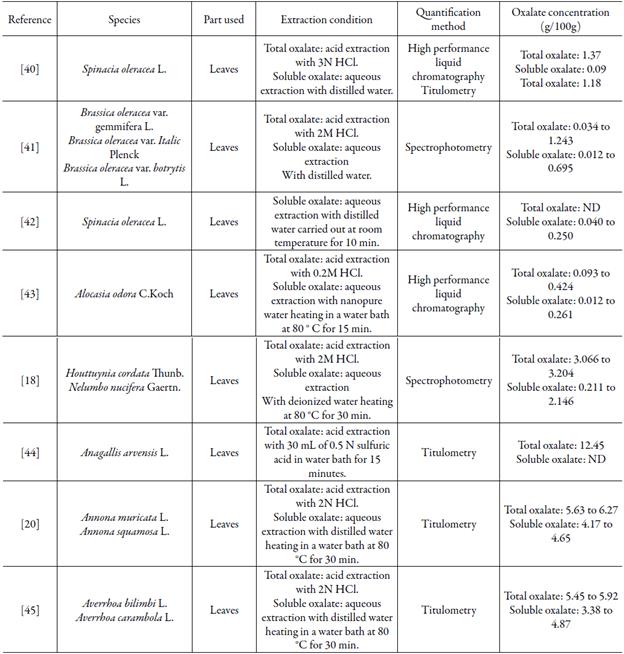
Wilson et al. [40] determined the concentration of oxalic acid through high performance liquid chromatography in Averrhoa carambola L., in 15 different cultivars in Florida, United States and found results that varied from 0.08 to 0.73 g/100 g. On another study Huang et al. [18], assessed through enzymatic method followed by spectrophotometry, the oxalic acid content in 22 species commonly used in traditional Chinese medicine, such as Houttuynia cordata Thunb. and Nelumbo nucifera Gaertn. These authors found the highest values of 3.204 g/100 g of total oxalate for Houttuynia cordata and 3.066 g/100 g of total oxalate for N. nucifera.
Mishra et al. [44], through titration and spectroscopy, determined the oxalate content contained in Anagallis arvensis L. finding the result of 12.45 g/100 g of dry vegetable drug. Sá et al. [20] determined through titration, the oxalic acid content in leaves of species used in treatment for chronic non-communicable diseases and found the amount of 5.64 g/100g of dry vegetable matter for Annona muricata L. and 6.27 g/100 g for Annona squamosa L. On another research, Sá et al. [45], also determined through titration, the concentration of oxalic acid in leaves of species used in treatment of diseases such as diabetes and colic. The authors found the value of 5.45 g/100 g for Averrhoa bilimbi and 5.92 g/100 g for Averrhoa carambola.
Oxalic acid is present in many leafy vegetables and plant foods. Depending on the species, oxalic acid can occur as soluble salts of potassium and sodium or as insoluble salts of calcium, magnesium or iron, or it still can occur as a combination of soluble and insoluble salts [46]. Insoluble salts are excreted in the feces, but soluble salts are absorbed by the organism, allowing the formation of strong chelates with calcium from the diet, inhibiting its absorption [47].
The high intake of oxalic acid and its salts can have a deleterious effect on nutrition and human health, mainly by decreasing the absorption of calcium and because it induces the formation of kidney stones. Thus, it is possible to assume that diets rich in oxalic acid need mineral supplementation to avoid nutritional deficiencies [48].
The optimization of processes allows identifying which activities do not add value to the final result and gives the possibility of eliminating them, without negative consequences. In addition, with the comprehension of the tasks and the assessment of alternatives, it becomes possible to develop methods for obtaining fast, reliable, low-cost and safer results [49, 50].
Of the analyzed species in this study, the ones that showed the highest results for oxalic acid concentrations were Psidium guajava and Artocarpus heterophyllus in their acid extract, therefore they were submitted to the optimization process. The oxalic acid extraction processes from Psidium guajava and Artocarpus heterophyllus leaves were submitted to univariate data analysis, in order to optimize the process and to provide information about the influence that each of the independent variables has on the process separately.
According to figure 2, the ideal parameters of time, temperature and concentration of the extracting solvent were obtained in the moment when there was not a significant difference in the concentration of oxalic acid, even with a rise in any of these parameters, thus obtaining a plateau in the optimization curves. As it is a univariate optimization, in which each step was incorporated into the previous one, on a continuous basis, it was not possible to statistically evaluate the isolated influence of each independent variable on the extraction yield.
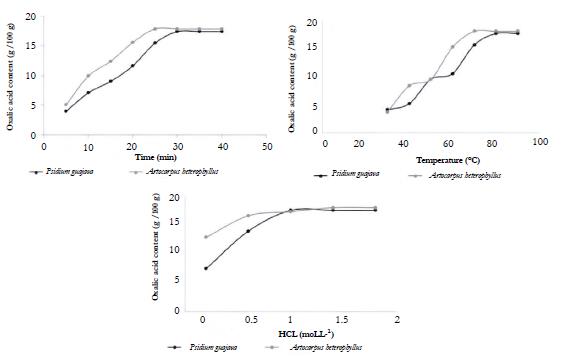
Figure 2 Graphs for univariate optimization referring to the extraction time (A), extraction temperature (B), and extraction solvent concentration (C) for the species Psidium guajava and Artocarpus heterophyllus.
The ideal extraction time for P. guajava was 30 minutes and for A. heterophyllus it was 25 minutes. The ideal extraction temperatures for P. guajava and for A. heterophyllus were 80 °C and 70 °C, respectively. The ideal hydrochloric acid concentrations for P. guajava and A. heterophyllus were 1.0 mol.L-1 and 1.4 mol.L-1, respectively.
Previous studies have already demonstrated that time has a direct influence on the extraction rate, since the longer the extraction time, the greater the interaction between the solvent with the analyte of interest is, favoring its extraction until there is no more concentration gradient that allow molecular diffusion [51, 52]. In this sense, the ideal extraction time for Psidium guajava was 30 minutes and for Artocarpus heterophyllus, 25 minutes. Most studies have a time that range from 15 to 30 minutes, under different conditions of temperature and pressure [17, 53].
Regarding temperature, the increase in it favors the extraction process since there is a decrease in the viscosity of the medium and an increase in the degree of molecular entropy, which benefits the process of molecular diffusion according to Fick's first law [54]. Therefore, according to figure 2, the ideal extraction temperatures for Psidium guajava and for Artocarpus heterophyllus were 80 °C and 70 °C, respectively. Most studies referring to the extraction and quantification of oxalic acid in plant species use extraction temperatures ranging from 60 °C to 100 °C [17, 53].
Although hydrochloric acid is widely used in pharmacobotany to identify oxalate crystals [21, 22, 23] it is also widely used in the oxalic acid extraction process as extraction solvent. In this study the ideal hydrochloric acid concentrations for Psidium guajava and Artocarpus heterophyllus were 1.0 mol.L-1 and 1.4 mol.L-1, respectively. Some of previous studies used a standard 2 N hydrochloric acid concentration that is equivalent to 2.0 mol.L-1 [24, 55].
Since oxidizable species could be interfering in the concentration calculation and changing the real value of oxalic acid contained in plant drugs, precipitation is an alternative to circumvent this problem of non-selectivity. The results of the oxalic acid concentration obtained from Psidium guajava and Artocarpus heterophyllus were 4.59 ± 0.086 and 5.21 ± 0.051 (g/100 g), respectively, demonstrating that there was a significant decrease in the oxalic acid content. In addition, polarized light microscopy performed on precipitated extracts of Psidium guajava and Artocarpus heterophyllus revealed the presence of calcium oxalate crystals (figure 3).
CONCLUSION
Although the use ofplant species to treat diseases is a very common practice, its use needs caution. The present study demonstrated that these studied plant species have significant amounts of oxalic acid. The results found for the 11 plant species can support medical decisions and professional prescribing of medicinal plants and herbal medicines, since in the literature a causal relationship between the consumption of oxalic acid and the formation of kidney stones is already evident, especially in patients who already have a predisposition, such as those with chronic non-communicable diseases.















