INTRODUCTION
Diabetes Mellitus (DM) is a syndrome with metabolic impairment of carbohydrates, fats and proteins. The World Health Organization states that the prevalence of type 2 diabetes (non-insulin dependent) has risen dramatically in countries of all income levels, type 2 is the most common. Complications represent one of the main causes of morbidity and mortality in the world [1]. The COVID-19 pandemic showed the prevalence and the severity of this disease, and its likely influence on the outcome of others [2]. Its origin is multifactorial and is related to genetic factors, diet and lifestyle [3]. Inflammation and oxidative stress are related to DM, therefore, the use of antioxidant substances is advocated, due to its potential for preserving beta cell function [4, 5].
In the diagnosis of this disease, parameters such as fasting blood glucose or post-load glucose are considered, and in type 2 diabetes the plasma insulin concentration can be high and increase severely in the glucose tolerance test [6, 7]. By and large, the control of type 2 diabetes mellitus (DM2) is performed by means of a strictly controlled diet, regular physical activity with body weight loss and, where needed, therapy with hypoglycemic agents [3]. Oral antidiabetics can act in different ways, increasing pancreatic insulin secretion (sulfonylureas, for example, glyburide and glinides), reducing glucose absorption (alpha-glucosidase inhibitors), reducing hepatic glucose production (biguanides), and increasing peripheral glucose use (glitazones). Glyburide is used together with exercise and diet to improve blood sugar control in adults with DM2, in addition, glyburide is not for treating type 1 diabetes [8]. The pharmacological approach to the treatment of diabetes mellitus is centered on achieving and maintaining glycemic control.
Although it is ideal to incorporate healthy habits it is known that part of the population seeks alternative ways to lose weight, such as fat burners [9]. Several supplements are advertised with the proposal of gaining lean mass. As they are over-the-counter products, they are likely to be used by healthy or diabetic individuals, including those who extensively use DM2 medications.
Chromium picolinate is a supplement that has been used for weight loss [10] and in rats it was effective in reducing the damage caused by oxidative stress, suggesting its potential beneficial effect in diabetic patients [11]. In the United States, it is more common to find capsules containing 200 fig, however, this dosage reaches up to 1000 fig, according to information obtained in Micromedex® in 2017. Carthamus oil (Safflower oil or Carthamus tinctorius) is a compound that has the potential to be used in numerous applications in food, cosmetic and pharmaceutical industries. This compound reduced the fetal malformation after supplementation of diabetic rats [12] and 200 mg/kg for 30 days exhibited a hypoglycemic effect in rats [13]. Other authors attribute Carthamus oil to an anti-inflammatory, antioxidant, immunomodulatory, neuroprotective effect, protection against myocardial ischemia and other pharmacological practices. In its composition there are components such as quinochalcones, flavonoids, alkaloids, polyacetylenes, fatty acids, steroids and lignans [14].
Considering the possibility that both Carthamus oil, chromium picolinate and glyburide may be hypoglycemic, the hypothesis of this research is that the concomitant use of these drugs can lead to a reduction in blood glucose. Thus, researching the effect of the association of these three substances and the intensity of this effect is important and requires in-depth studies, since they can be used freely by diabetics. Although there is some research on these substances in isolation, no studies were found to assess their interaction, therefore, the objective of this research was to evaluate the effects of chromium picolinate and Carthamus oil administered orally in diabetes-induced rats and their influence on the effectiveness of glyburide.
MATERIAL AND METHODS
Reagents
Carthamus oil and chromium picolinate were purchased from masterly handling pharmacy. Glyburide e streptozotocin were purchased from Cayman Chemical (Michigan, USA). Other reagents used in this study were of the highest quality available from commercial vendors.
Preliminary studies were carried out to define a vehicle for dispersion, which was added with Carthamus oil 15 mg/mL, glyburide 5 mg/mL and/or chromium picolinate 2.5 μg/mL. The excipients used were: Polysorbate 20 (1.25 mL), Cellulose Gum (1 g), Methylparaben and Propylparaben solubilized in Propylene Glycol (2.5 mL) and purified water (qsp 100 mL).
Effects of the formulations on rats
Twenty-four male 35-day Wistar rats (weight: 95.55 ± 16.45 g) were obtained from the Multidisciplinary Center for Biological Research in the Area of Laboratory Animal Science of the University of Campinas (SP, Brazil) and maintained at 21 °C ± 2 °C with a 12-h artificial light cycle, with free access to tap water. All animals were allowed to acclimatize for a week, followed by randomization and allocation [15].
All procedures were conducted in accordance with the Brazilian guidelines on animal experimentation of the National Council for the Control of Animal Experimentation (Concea) and approved by the Committee on Ethics in the Use of Animals of Pontifical Catholic University of Campinas under the number 013/2017.
Diabetes induction
Diabetes was induced in overnight fasted rats by an intraperitoneal injection of freshly made solution of STZ (50 mg/kg) in sterilized 100 mM citrate buffer, pH 4.5. After three days, fasting capillary glycemia was determined [16, 17].
Treatments
The rats were randomized in four groups of 6 animals each. The treatments were administered by gastric gavage once a day for 10 days at 9 a. m.: G1: healthy group and received saline; G2: diabetic group and received 10 mg/kg glyburide; G3: diabetic group and received 30 mg/kg/day Carthamus oil and 5 μg/kg/day chromium picolinate and G4: diabetic group and received 10 mg/kg glyburide, 30 mg/kg/day Carthamus oil and 5 μg/kg/day chromium picolinate.
Capillary glycemia and enzyme determination
After the 10 days experimental period and a 12 h fasting period, blood samples from the caudal vein were used to determine fasting blood glucose. Then, gavage was performed with 0.5 ml of 20% sucrose solution and after one hour the glucose was reevaluated. The animals were anesthetized with ketamine (75 mg/kg) associated with xylazine (10 mg/kg) intramuscularly and blood was collected by direct cardiac puncture for the collection of 4 mL from each animal [18].
The levels of hepatic transaminases AST and ALT, total cholesterol and triglycerides were determined in the blood serum by using commercially available kits (Labor-LabTM). Urine samples were analyzed with reagent strips. For the determination of blood glucose, a glucometer (AccuChekTM, Roche Diagnostics) was used. After the above procedures, the animals were euthanized with excess anesthesia [5, 17].
RESULTS AND DISCUSSION
In the clinical evaluation of the animals, higher water consumption was perceived in groups G2 to G4, however, as it was not quantified, it was not possible to analyze statistically. All gained weight over the study period, with no statistical difference between groups. The results of the urine analysis were normal in all parameters, highlighting the absence of glucose.
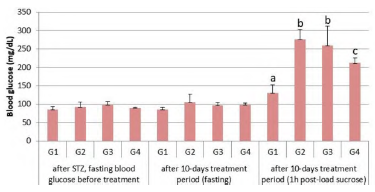
Figure 1 Blood glucose (mean ± SE, n = 6). G1: Control group, G2: Diabetic group treated with glyburide, G3: Diabetic group treated with Carthamus oil and chromium picolinate, G4: Diabetic group and received glyburide, Carthamus oil and chromium picolinate. Different letters indicate significant differences (p < 0.05).
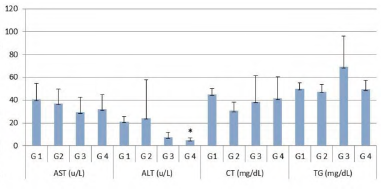
Figure 2 Dosages of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC) and triglycerides (TG) of rats after 10-days treatment period. G1: Control group, G2: Diabetic treated with glyburide (10 mg/kg), G3: Diabetic group treated with Carthamus oil (30 milligrams/kg/day) and chromium picolinate (5 μg/kg/day), G4: Diabetic group and received 10 mg/kg glyburide, 30 mg/kg/day Carthamus oil, and 5 μg/kg/day chromium picolinate. *Statistical analysis: Kruskal-Wallis test (p < 0.05).
There was no significant difference in the results obtained for fasting glucose from rats treated with STZ compared to healthy ones. However, after sucrose intake, there was an increase in blood glucose, as expected, due to damage to insulin-secreting cells by the effect of STZ (figure 1). The increase in blood glucose after sucrose intake was less evident in the group that received the interaction of glyburide, Carthamus oil and chromium picolinate, although it also occurred in the other groups. Thus, the comparison between G1 and G2 indicated significance (p < 0.0001) as well as between G1 and G4 (p < 0.05), with no difference between G2 and G3 (p > 0.05).
There was a reduction in ALT (p < 0.05) only for the diabetic group that received Carthamus oil + chromium picolinate + glyburide (G4). There was no difference in the values of AST, TC and TG (figure 2).
A topic that has received high attention in recent times is nutritional supplementation for different purposes and improvement of the general aspect of the human body. As quoted by Catic and Jusufovic [19]: "food or dietary supplements are becoming more and more used over past 20 years and it becomes a matter of consumer interest". Given that they are over-the-counter products, they are likely to be used by healthy or sick individuals, including those who regularly use type 2 diabetes mellitus medications. However, diabetic individuals can consume herbal compounds along with their prescription medications, which could lead to an important interaction that is not described in the literature [20]. An important interaction was found by Abdel-Kader et al. that demonstrated that 500 mg of olive leaf extract increases the antidiabetic effect of glyburide in diabetic rats [21].
The interactions can be of a pharmacodynamic or pharmacokinetic nature. If an herbaceous plant alters the expected pharmacological effect because of its biochemical or physiological effect, this is known as a pharmacodynamic interaction. In case of the same pharmacological effect, there may be an intensification of the therapeutic effect with its co-administration. Dose adjustment would probably be necessary, otherwise the resulting effect could be harmful if monitoring and evaluation are not done.
In the present study, fasting blood glucose from rats that received streptozocin (G2, G3 and G4) was considered normal and without statistical difference when compared to the control group (G1), at the beginning and again after 10 days of treatment (figure 1). This result may explain the absence of glycosuria in all groups. However, it can be stated that pancreatic injury did occur, a fact that was confirmed after the marked and significant elevation of blood glucose in animals after oral sucrose, what will be discussed ahead.
Such data corroborate with Kumar et al. [6] who carried out a review on diabetes diagnosis. These authors indicate that in order to diagnose DM, it's necessary to taken account a) fasting blood glucose is investigated, which should not exceed 7 mmol/L, a value that was not exceeded by any group and that could indicate the absence of pancreatic injury and b) post glucose, which should not exceed 11.1 mmol/L, which was exceeded by the groups that received sucrose solution, namely, for G4 the average value found was 11.8 mmol/L, for G2 it was 15.4 mmol/L and G3 showed 14.4 mmol/L, against 7.3 mmol/L that was obtained in the G1 control group. In this case, we could say that animals have become prediabetic, a condition that increases the risk of various complications, such as retinopathies, nephropathies and risk of cardiovascular diseases [6].
Some authors mention that the time between dilution and injection of STZ in animals immediately interferes with toxicity, in other words, the activity on beta cells. In our study, the interval between dilution and use was about 30 minutes, depending on the experimental conditions. Thus, although the dose used is adequate, this interference possibly occurred.
Our findings indicated that the treatments influenced the glycemic level, especially the association of Carthamus oil, chromium picolinate and glyburide. It was found that the blood glucose levels after sucrose in the glyburide group (G2) were higher than in the group (G1) withp < 0.0001; and a similar result was obtained for the group Carthamus oil, chromium picolinate and glyburide (G4) withp < 0.05, as the group having the lowest blood glucose values. It was found that there was no difference between groups G2 and G3 post sucrose, indicating that such treatments had equivalent effects, which were intensified when the three compounds were used together in G4. These results indicate that probably the joint use of such compounds can contribute to the reduction of the level of glucose in the bloodstream after the ingestion of carbohydrates by individuals, who have disorders in glucose uptake by cells and/or pancreatic injury.
In a study conducted by Pala et al. [22], there is a demonstration that supplementation with chromium picolinate may favor the expression of glucose transporters, which would be an indication of why the groups which received treatment with chromium picolinate present lower blood glucose results than the group which received only glyburide after the administration of sucrose solution at the end of the experiment.
The assessment of liver enzymes, cholesterol and triglycerides aimed to verify whether the proposed interaction could interfere with liver function and circulating lipids. There was a significant reduction in ALT values in the G4 group in relation to the control (p <0.05) and this result is important, as the dosage of this enzyme is used, along with other parameters, to verify liver damage, thus suggesting that treatment performed would be contributing to hepatic homeostasis (figure 2). These findings are in agreement with Moreira et al. [23], who described that chromium picolinate alone reduced liver enzymes, as well as glycemia and lipid profile in diabetic rats.
No differences were found among the AST, TC and TG values of the groups that received treatment and the control group (figure 2). Even if DM leads to oxidative stress, some of these parameters could have changed, which did not occur probably due to pancreatic injury not being too much or oxidative stress not having been established during the study period, which may be considered as a limitation of our study.
Although Carthamus oil and chromium picolinate are compounds widely disseminated and used by the population, there are significant results in studies carried out with Carthamus oil in humans [24] and chromium picolinate in rats [22], we have however not found any other study that has associated them in a similar context, thus presenting an unprecedented study.
It should be noted that even if the treated rats showed glycemic disorders, the results obtained in the present study should not be extrapolated without a previous study in humans. Therefore, regarding diabetic individuals, it can be suggested that this treatment is promising, however it requires dosage adjustments and study for a prolonged period, but apparently does not interfere with the lipid profile and is not hepatotoxic.
In a general way, the associated use of chromium picolinate and Carthamus oil was favorable to the action of glyburide. Therefore, more studies can be carried out by extending the time of use and carrying out studies in humans, in addition to the need to combine with other antidiabetics to assess their safety and effectiveness.
CONCLUSION
This study involved the combination of Carthamus oil (30 mg/kg) and chromium picolinate (5 ig/kg) interacted with glyburide (10 mg/kg) in treatment for ten days, which contributed to the reduction of blood glucose and serum ALT levels in pre-diabetic rats, being promising for future investigations in humans.















