INTRODUCTION
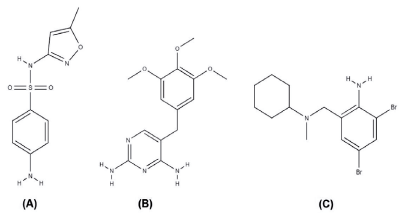
Sulfamethoxazole (SMT), chemically called 4-amino-N-(5-methyl-3-isoxazolyl) benzenesulfonamide, has a molecular formula C10H11N3O3S and a molar mass of 253.28 g-mol-1 (figure 1A) [1], it is an antimicrobial that belongs to the class of sulfonamides, often used in combination with the drug trimethoprim (TMP) in pharmaceutical formulations [2].
TMP, chemically known as 2,4-diamino-5-(3',4',5'-trimethoxybenzyl)-pyrimidine, has a molecular formula C14H18N4O3 and a molar mass of 290.32 g-mol-1, is a drug of the class pyrimidines that have antimalarial and antibacterial activity (figure 1B) [1, 3]. The association of SMT and TMP was introduced to the market in the late 1960s, for presenting synergistic effect and greater potency, in relation to the individual use of these agents by blocking enzymes that catalyze sequential steps in the pathway of folic acid metabolism in bacteria, which can be administered to humans and animals [3-5]. In veterinary medicine, the drugs SMT and TMP can also be found in association with the drug bromhexine (BMX). BMX, chemically called 2,4-dibromo-6-[[cyclohexyl (methyl) amino] methyl] aniline, has molecular formula C14H20Br2N2 and molar mass of 376.1 g-mol-1. BMX is a mucolytic drug used in the treatment of respiratory tract disorders (figure 1C) [1, 5].
To determine the association of SMT and TMP, several analytical methods have been developed and are described in the literature: capillary electrophoresis [6], micellar electrochromatography [7], differential pulse voltammetry [8], UV-visible spectrophotometry [9-14], liquid chromatography (LC) [15-17], high performance liquid chromatography, (HPLC) [18-21]. However, no analytical method has yet been reported in the literature for the simultaneous determination of SMT, TMP and BMX.
Therefore, the objective of the present study was to develop and validate a simple, accurate and fast analytical method for simultaneously determining the association of SMT, TMP and BMX in pharmaceutical formulation for animal use.
MATERIAL AND METHODS
Standard reference substances and solvents
The drugs SMT (purity 100%) and TMP (purity 99.24%) were purchased from the pharmaceutical laboratory FURP and BMX (purity 99.4%) was purchased from the distributor Formil Química used as standard reference substances were accompanied by the certificate of analysis of the providers. The commercial sample used during the development and validation of the analytical method was Sulfaforte® (SMT 30.0 g + TMP 6.0 g + BMX 1.0 g every 100 g, donated by Evance Saúde Animal). The sample was chosen because it is widely used in veterinary medicine for the treatment of poultry and pigs. The solvents used were: ultrapure water, obtained from a Milli-Q Plus® system and methanol (analytical grade).
Equipment and chromatographic conditions
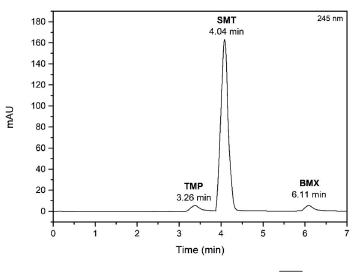
The method was developed and validated in an Ultimate 3000 high-performance liquid chromatograph (Thermo Fisher Scientific, USA), equipped with a diode array detector (DAD) in the 200 to 400 nm range and a quaternary pump. The chromatographic separation occurred in the isocratic mode at a flow rate of 0.7 mL-min-1 on an AcclaimTM120 column (4.6 x 250 mm, 5 μm) from ThermoScientific®. The mobile phase was composed of methanol and water (84:16 v/v), pH adjusted to 3.0 with phosphoric acid. Detections were performed at 265 nm for SMT, 271 nm for TMP and 245 nm for BMX. The injection volume was 20 μL and the running time was less than 7 min. The analyzes were performed at room temperature (25.0 ± 1.0 °C).
Validation of the HPLC-DAD method
The analytical parameters of linearity, limit of detection (LD), limit of quantification (LQ), precision, robustness, accuracy, selectivity were evaluated for validation of spectrophotometric methods according to the Guide for validation and analytical quality control of drugs in products for food and veterinary medicines [22] and RDC 166/2017 [23] and guides Internacional Conference on Harmonization [24] and Association of Official Analytical Chemists International [25]. The results were analyzed statistically using Microsoft Excel® (Microsoft, Washington-USA) and OriginPro 9.0® (OriginLab, Northampton-UK). The standard SMT, TMP and BMX stock solutions at 100 μg-mL-1 were prepared separately in methanol and homogenized in an ultrasonic bath for 15 min . Subsequently, each standard solution was diluted using methanol: water (84:16, v/v, pH 3.0) in different concentrations for validation of the analytical method were prepared, as described below.
Preparation of standard stock solutions and commercial sample
Standard stock solutions were prepared weighing analytically 10 mg of each Standard reference substances (SMT, TMP and BMX) and commercial sample that were transferred separately to 100 mL volumetric flasks, adding 50 mL of methanol. Then, these solutions were taken to the ultrasonic bath for 15 min. Subsequently, the volumes were made up to 100 mL with methanol, obtaining solutions at 100 μg-mL-1 of each drug and commercial sample (SMT 30 μg-mL-1, TMP 6 μg-mL-1 e BMX 1 μg-mL-1), these concentrations were chosen because they are proportional to the composition of drugs in the commercial sample (SMT (30.0 g), TMP (6.0 g) and BMX (1.0 g) ) for each 100 g.
Analytical parameters
Linearity
It was evaluated by building three analytical curves for each drug. The analytical curve for SMT was obtained in the concentration range of 15.0 to 45.0 μg-mL-1, for TMP in the range of 3.0 to 9.0 μg-mL-1 and for BMX in the range of 0.5 at 2.0 μg-mL-1. The determinations were carried out in triplicate and the results were submitted to linear regression analysis to obtain the analytical curves, line equations and correlation coefficients for each drug.
Limit of detection (LD) and limit of quantification (LQ)
The LD and LQ of SMT, TMP and BMX were determined from three analytical curves obtained for each drug, using the standard deviation of the intercept (SD) and the mean slope (a). Detection limit estimation can be done with based on the ratio of 3 times the baseline noise and, in the case of the quantification limit, the baseline noise is also determined, considering the concentrations that produce a signal-to-noise ratio greater than 10:1. Equations (1) were used to calculate LD and LQ:
Where: LD is limit of detection, LQ is limit of quantification, DP is standard deviation, and a is slope of calibration curves.
Precision
The precision of the method was assessed by intra-day (repeatability) and inter-day (intermediate precision) tests. Repeatability was performed by analyzing six determinations of the stock solution of the commercial sample, under the same chromatographic conditions and by the same analyst. Intermediate precision was performed by analyzing the commercial sample by two analysts on three different days, at the concentrations mentioned above. The relative standard deviation (RSD) was calculated from the results obtained.
Robustness
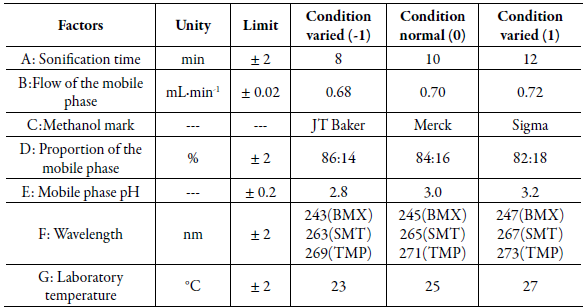
Robustness was assessed by the Plackett-Burman factorial model, where seven parameters that can interfere with the analytical result were changed, to analyze the influence of these variations. The selected parameters were: sonication time, flow and proportion of the mobile phase, methanol supplier, pH of the mobile phase, wavelength and laboratory temperature. The letters A to G represent the selected parameters, the numbers 1 to 15 represent the number of experiments, where the level (0) represents the normal conditions of the method, while the levels of (1) and (-1) are , respectively, the upper and lower values in relation to normal conditions (0). The tested parameters and levels are shown in table 1.
Table 1 Selected factors and levels of variation used in robustness, according to the Plackett-Bur-man factorial model.
SMT: sulfamethoxazole; TMP: trimethoprim; BMX: bromhexine.
The influence of the variations of each parameter in the final result was determined by the average of the results obtained in the assays with the normal parameters in comparison with the average corresponding to the altered parameters, and the effect generated by each variable, which is the difference between the results obtained in normal conditions and changed parameters. Table 2 presents the factorial combination used in the Plackett-Burman test, where the letters A to G represent the selected parameters; numbers 1 to 15 represent the number of experiments, where level (0) represents normal method conditions, while levels (1) and (-1) are, respectively, the higher and lower values in relation to normal conditions (0).
Table 2 Plackett-Burman factorial combination applied in the robustness test.
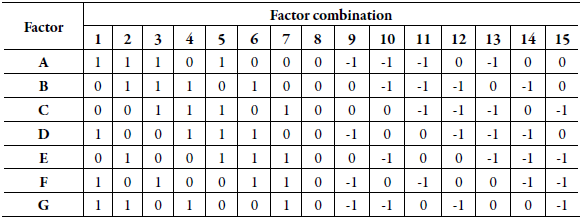
A-G: selected factors; 1-15: number of experiments.
The deviation produced by each factor was calculated using the Youden and Steiner methodology [26]. Equation (2) demonstrates the evaluation of the effect caused by the change in variable A (sample sonification time) and the other factors were also evaluated by this equation.
where: S =
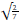 (DA2 + DB2 + DC2 + DD2 + DE2 + DF2 + DG2) (2)
(DA2 + DB2 + DC2 + DD2 + DE2 + DF2 + DG2) (2)
The method will be considered robust if the effect on each parameter (DA, DB, DC, etc.) is less than the critical value resulting from the V2S [27].
Accuracy
The accuracy was determined through recovery tests by adding known quantities of standard solutions to the commercial sample solution [25]. The drugs were analyzed individually according to the concentration linearity range. For this purpose, the standard stock solutions of the drugs and the commercial sample, described above, were used. The percentages of recoveries for each drug were calculated according to equation (3) [25]:
where: R is recovery, Ca is the drug concentration found in the standard added sample (μg-mL-1), Cna is the drug concentration found in the standard non-added sample (μg-mL-1) and Ctp is the theoretical standard concentration added to the sample (μg-mL-1).
Selectivity
It was verified by assessing the interference of adjuvants in the quantification of drugs present in the commercial sample under study, in relation to the analytical methodology developed. For this, a placebo solution containing the mixture of the following adjuvants was prepared: lactose (65%), starch (10%), cellulose (5%) and ultrapure water (q.s.p. 100 mL). The chromatogram of the commercial sample was compared with the placebo chromatogram.
Forced degradation study
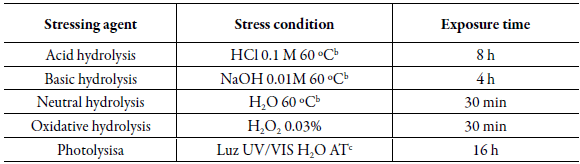
The forced degradation study was carried out in accordance with the recommendations of the international drug and pharmaceutical stability study guides [28, 29] and the experimental protocol developed by Sversut et al. (2019) [30]. For this, aliquots of the solution of the raw materials in their combined form, that is, SMT, TMP and BMX, were subjected to stress conditions, such as acid, alkaline, neutral, oxidative and photolytic hydrolysis. From a standard stock solution of 5000 μg-mL-1, solutions were prepared with a mixture of methanol:water (84:16, v/v, pH 3.0), adjusted with phosphoric acid, to reach a final concentration of 30.0 μg-mL-1 for SMT, and 6.0 μg-mL-1 for TMP and 1.0 μg-mL-1 for BMX.
The resulting solutions were inj ected in triplicate and analyzed using the proposed chromato -graphic method: C18 AcclaimTM120 column (4.6 x 250 mm, 5 μm) from ThermoScien-tific®, mobile phase methanol and water (84:16 v/v, pH 3.0 ), adjusted with phosphoric acid, isocratic mode, flow rate of 0.7 mL-min-1. In order to carry out the forced degradation study, experimental conditions that promoted an average degradation of10 to 30% for each of the drugs were defined as ideal [31]. Table 3 shows the degradation conditions that were tested.
Method Applica bility
The commercial sample Sulfaforte® was analyzed using the proposed analytical method. The sample-stock solution was prepared as previously described, obtaining the following theoretical concentrations: SMT 30 μg-mL-1, TMP 6 μg-mL-1 and BMX 1.0 μg-mL-1. The content of each drug present in the commercial sample was calculated using analytical curves.
RESULTS AND DISCUSSION
Method development
After conducting tests with several chromatographic conditions: columns, solvent mixtures, flow and pH of the mobile phase, the method for the simultaneous determination of SMT, TMP and BMX was developed with the conditions that produced the best results in the parameters: resolution, asymmetry, plates and peak purity, ensuring system compliance and data quality, indicating system selectivity, column accuracy and efficiency [32].
Table 4 shows the chromatographic conditions selected for the development and validation of the analytical method: AcclaimTM120 column (4.6 x 250 mm, 5 fim) from ThermoScientific®, flow rate of 0.7 mL-min-1 and mobile phase composed of methanol:water (84:16 v/v) with pH adjusted to 3.0.
Table 4 Parameters evaluated in the compliance analysis of the chromatographic system with the AcclaimTM120 column (4.6 x 250 mm, 5 fim) from ThermoScientific®.
| Drug | Evaluated Parametersa,b | ||||
|---|---|---|---|---|---|
| Retention time (min) | Resolution | Asymmetry | Plates | Purity | |
| Sulfamethoxazole | 4.04 ± 0.16 | 5.65 ± 0.35 | 1.09 ± 0.42 | 3680 ± 0.24 | 996 ± 0.31 |
| Trimethoprim | 3.26 ± 0.19 | 3.38 ± 0.17 | 1.26 ± 0.65 | 4589 ± 0.21 | 998 ± 0.10 |
| Bromhexine | 6.11 ± 0.15 | - | 1.29 ± 0.71 | 4337± 0.43 | 968 ± 0.79 |
a Conditions used: mobile phase composed of metanol:water (84:16 v/v) pH 3.0 and flow rate of 0.7 mL-min-1. b Mean ± coefficient of variation of 6 determinations.
After choosing the column, mobile phase and flow for the analysis of the drugs and development of the analytical method, the wavelengths for the quantification of the drugs were selected. Spectral scans were performed with the aid of the diode array detector (DAD) in the range 200-400 nm. The wavelengths selected for SMT, TMP and BMX quantification were 265 nm, 271 nm and 245 nm, respectively, corresponding to the regions of maximum UV absorption of each drug (figure 2).
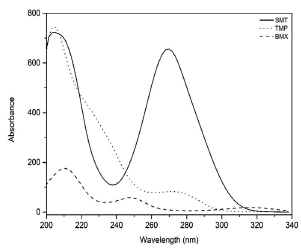
Figure 2 Absorption spectrum in the UV region of the drugs sulfamethoxazole (SMT) (30 μg-mL-1), trimethoprim (TMP) (6 μg-mL-1) and bromhexine (BMX) (1 μg-mL-1) in methanol:water (84:16 v/v, pH 3.0).
Figure 3 shows the chromatogram of the chromatographic separation of the solution of the drugs SMT, TMP and BMX, under the developed chromatographic conditions. In figure 3 is possible to verify the good chromatographic separation of the three drugs, in a short time of analysis (7 min), indicating that the method is suitable for validation and simultaneous determination of drugs in pharmaceutical formulations for animal use.
Method validation
Linearity, LQ and LD
Table 5 describes the equations of the line, the correlation coefficients and LQ and LD obtained from the analytical curves of the drugs under study.
Table 5 Results obtained on the calibration curves performed in the linearity test, limits of detection and quantification.
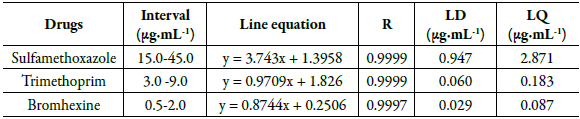
R: correlation coefficient; LD: detection limits; LQ: quantification limits.
The analytical curves were linear, as they presented correlation coefficients close to 1.0 (r > 0.9997) in the concentration range used for drug analysis, demonstrating that the results obtained were directly proportional to the concentration of the analyte in the sample, within the specified interval. The LD and LQ values indicated that the proposed method, since it can determine small concentrations of the analyzed drugs, demonstrating to be sensitive for simultaneous determination of drugs in pharmaceutical formulations.
Table 6 describes the results of the statistical analysis performed by the Anova test, in which it was possible to observe that the linear regression was significant for the drugs analyzed (Fcalculated> Critical), therefore, the concentration of the analytes is correlated with the signal generated by the equipment (peak area).
Table 6 Analysis of variance (Anova) of the linear regression of the drugs sulfamethoxazole, tri-methoprim and bromhexine.
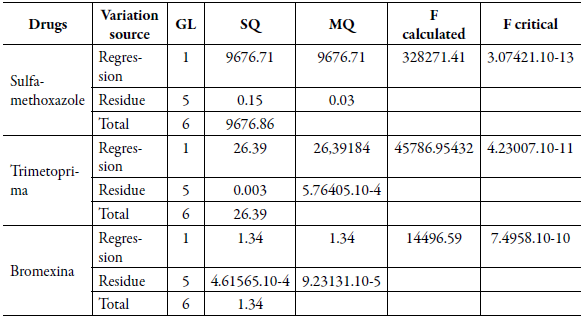
GL: Degree of freedom; SQ: Sum of squares; MQ: Mean of squares.
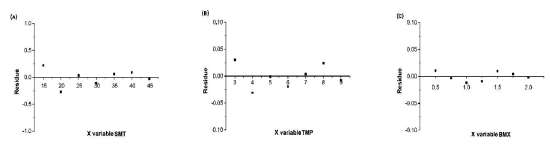
In addition to the analytical curves and the Anova test, linearity can also be verified through the regression analysis of residues. In this analysis, it was possible to verify the linearity of the method from the linear regression of the drugs under study, which were distributed in the graphs homogeneously, close to the zero axis, indicating the homoscedasticity of the variances (figure 4).
Precision
The results obtained in the intermediate precision and repeatability of the drugs under study are shown in table 7. In the determinations made with SMT, TMP and BMX, it was possible to verify that the analyzed drugs had DPR below the maximum recommended limit of 2.0%, confirming the precision of the analytical method developed [24, 33].
Table 7 Results obtained in the precision parameter of the analytical method.
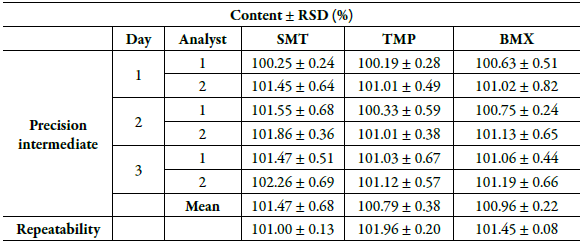
RSD: relative standard deviation; SMT: sulfamethoxazole; TMP: trimethoprim; BMX: bromhexine. Robustness
Figure 5 shows the absolute values of the effects observed in the robustness test. The critical values (V2S) in relation to the levels of SMT, TMP and BMX for the upper levels were, respectively, 1.26, 1.36 and 1.32 (figure 5A), and for the lower levels, 1.33 , 1.37 and 1.22 (figure 5B).
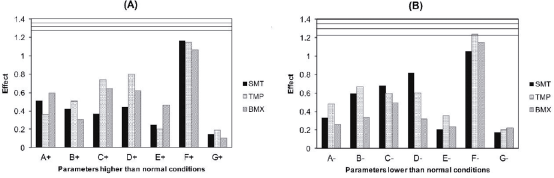
Figure 5 Critical effects and values of the levels of sulfamethoxazole (SMT), trimethoprim (TMP) and bromhexine (BMX) by the Plackett-Burman test. (A) Conditions higher than normal (1): the lines represent the critical values 1.26, 1.36 and 1.32, for the SMT, TMP and BMX drugs, respectively. (B) Conditions lower normal (-1): the lines represent the critical values 1.33, 1.37 and 1.22, for the SMT, TMP and BMX drugs, respectively.
Of the modified conditions, none significantly influenced the contents of the drugs, not exceeding the critical values, as well as in the retention times, asymmetry, resolution and number of plate. Although the factors analyzed did not significantly influence the quantifications, the variations made in the conditions inferior and superior to the normal ones in the wavelength factor, were those that presented greater effects in relation to the other factors. Therefore, the method proved to be robust for simultaneous determination of SMT, TMP and BMX, being able to resist small variations in the selected parameters, since the effects on the parameters were less than the critical values.
Accuracy
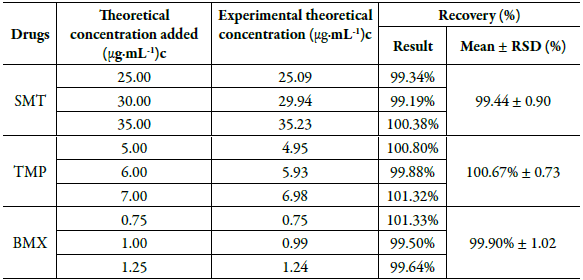
The accuracy of the method was determined through the recovery tests. Table 8 shows the values obtained in the recovery test. The average percentages of SMT, TMP and BMX recovery were 99.44%, 100.67% and 99.90%, respectively.
Table 8 Accuracy test of the drugs sulfamethoxazole, trimethoprim and bromhexine, analyzed by the proposed chromatographic method.
RSD: Relative standard deviation; SMT: Sulfamethoxazole; TMP: Trimethoprim; BMX: Bromhexine.
Therefore, the recovery values obtained from the drugs were within the acceptance limits of 98 to 102% for the analyzed concentration levels [25], indicating that the proposed method has accuracy for the simultaneous determination of SMT, TMP and BMX.
Selectivity
Figure 6 shows the chromatogram of the placebo solution overlaid with the chromatogram of the commercial sample solution. The adjuvants used for selectivity analysis did not interfere with the simultaneous determination of SMT, TMP and BMX, since the chromatogram did not show peaks eluting at the same retention time of the analyzed drugs and the peak purity was above 950, indicating that it did not there was coelution with other substances. Therefore, this result shows the selectivity of the method developed for simultaneous analysis of SMT, TMP and BMX in pharmaceutical formulations for animal use.
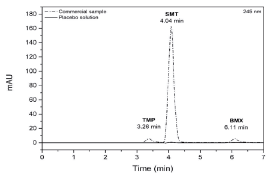
Figure 6 Chromatogram of the adjuvant placebo solution superimposed on the chromatogram of the commercial sample solution prepared in methanol:water (84:16 v/v), analyzed using the proposed chromatographic method: mobile phase methanol:water (84:16 v/v) with pH 3.0 and flow rate of 0.7 mL min-1 at 245 nm. SMT: sulfamethoxazole; TMP: trimethoprim; BMX: bromhexine.
Therefore, this result shows the selectivity of the method developed for simultaneous analysis of SMT, TMP and BMX in pharmaceutical formulations for animal use.
Forced degradation study
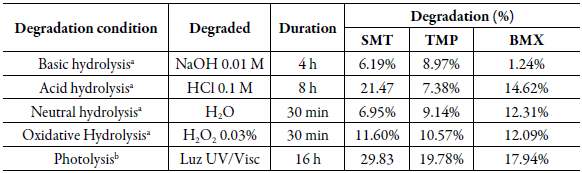
In the forced degradation study, the drug degradation percentages were determined, under the conditions selected for the degradation study of the association SMT, TMP and BMX. In table 9, it is possible to observe that the drugs had different percentages of degradation, and not all conditions allowed the degradation within the recommended limit (10 to 30%) [31].
Table 9 Percentage of drug degradation, under the conditions selected to carry out the forced degradation study of sulfamethoxazole (SMT), trimethoprim (TMP) and bromhexine (BMX).
aExposure temperature: a 60°C e bAmbient temperature (25 ± 2°C); clamps (UVB: 290-350 nm) and (Vis: 400-700 nm).
The chromatograms of the association of SMT, TMP and BMX, obtained from the method developed and validated in the forced degradation study, are illustrated in figure 7.
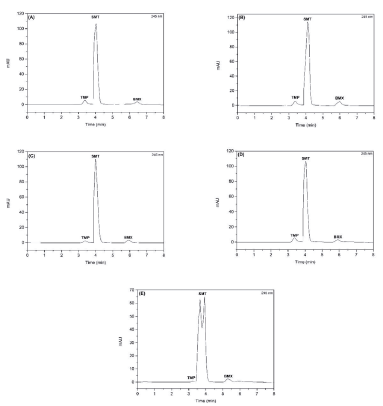
Figure 7 Chromatrograms of the association of sulfamethoxazole (SMT), trimethoprim (TMP) and bromhexine (BMX) (A) acid hydrolysis (HCl 0.1 M, 8 h); (B) alkaline hydrolysis (0.01 M NaOH, 4 h); (C) neutral hydrolysis (H2O, 30 min); (D) oxidative hydrolysis (0.03% H2O2, 30 min) and (E) photolysis (UV/VIS light, 16 h). Chromatographic conditions: AcclaimTM120 C18 column (4.6 x 250 mm, 5 μm) ThermoScientific®, mobile phase methanol:water (84:16 v/v), pH 3.0, flow rate of0.7 mL-min-1.
In acid hydrolysis (figure 7A), SMT, TMP and BMX degraded 21.47%, 7.38% and 14.62%, respectively. In this condition, only the TMP presented degradation below the minimum limit of 10%. Under alkaline hydrolysis (figure 7B), only BMX showed degradation above the minimum limit of 10%, SMT and TMP were degraded by 6.19% and 8.97%, respectively. Under oxidative conditions (figure 7D), SMT, TMP and BMX, degraded 11.60%, 10.57% and 12.09%, above the minimum limit of 10%.
Ghanem and Abu-Lafi (2013) [34] found similar results in acid and basic degradation tests for TMP at room temperature. Under acid hydrolysis conditions (HCl 1.0 N/ 60 min) TMP showed a degradation of 7.21%, in alkaline hydrolysis (NaOH 1.0 N/60 min) the degradation was 6.06% and under oxidative stress (10% H2O2/ 24 h) 23.27%, the highest percentage of degradation in relation to the other stress conditions they analyzed.
Wang, Zhang and Cai (2013), in the forced degradation analyzes of TMP identified under acid hydrolysis (HCl 1 N/ 5 h in a water bath) presented 21% degradation and under oxidative hydrolysis (H2O2 3%/ 2 h in a water bath) about 79% [17].
Tahan et al. (2015) observed in their study that in oxidative degradation (1 mol-L-1 H2O2) SMT only after 24 h and TMP after 1 h, presented degradation, with no interference peaks in the drug retention time [35].
In the condition of photolysis degradation (figure 7E), the results showed extensive degradation compared to other stress conditions. In this condition, the SMT presented a percentage of degradation of 29.83%, close to the maximum limit (30%), which can be attributed to the significant photodegradation of this drug.
The purity of the chromatographic peaks of the drugs was above 950 in the analyzed degradation conditions, indicating that the chromatographic peaks of the drugs did not present coelution of degradation products.
In addition, it was observed that there were no significant changes in drug retention times in the degradation test compared to drugs in non-degraded solutions. Therefore, the method developed and validated by HPLC-DAD can be considered indicative of stability for simultaneous determination of the association of SMT, TMP and BMX, as it enabled an efficient separation of drugs.
Method Applicability
The analytical method developed and validated by HPLC-DAD proved to be suitable for simultaneous determination, qualification and quantification of the association of SMT, TMP and BMX in pharmaceutical formulations for animal use. For the commercial formulation Sulfaforte® the levels found were 100.69 ± 0.17% SMT, 99.52 ± 0.13 TMP and 98.75 ± 0.26 BMX.
CONCLUSIONS
The results showed that the analytical method by HPLC-DAD, developed and validated for simultaneous determination of SMT, TMP and BMX in veterinary formulation, proved to be fast, selective, linear, precise, accurate and robust, being able to be applied for quality analyzes of this association in pharmaceutical industries in order to guarantee the safety and efficacy of medicines, and in the determination of these drugs in environmental and biological matrices.