INTRODUCTION
Due to the appearance of strains resistant to several of classic antibiotics, a great public health problem has been generated, whose result is that drugs become ineffective, and infections persist, increasing the risk of spreading to other human beings and the need to use new antibiotics [1].
Linezolid (ATC code J01XX08) is a drug of the fluorinated oxazolidinone class, structurally similar to furazolidone that acts by binding to a site in the 50S subunit of ribosome RNA and thus inhibits bacterial protein synthesis by interfering with trans-duction [2]. In Colombia, this antibiotic is indicated in the alternative treatment of infections when it is known or suspected that they are caused by susceptible organisms including those associated with concurrent bacteremia such as: community acquired pneumonia and nosocomial pneumonia. Skin and soft tissue infections, including diabetic foot infections, streptococcal infections, methicillin-resistant and sensitive Staphylococcus aureus infections, vancomycinresistant enterococcus infections, and in combination therapy if the presence of a Gram-negative bacteria is documented or suspected [3]
Resistance has been defined as the phenomenon in which the microorganism (e.g., bacteria, fungus or virus) is no longer affected by the antimicrobial to which it was sensitive. This phenomenon occurs naturally over time, usually through genetic changes, but can be accelerated by the inappropriate use of antimicrobials [4]. The use of antibiotics in medical procedures, management of infections, and other pathologies, surgeries and chemotherapy rise the risk of resistance, which may lead to an increase in the cost of health care, for instance, by requiring longer hospital stays and treatment in intensive care units (ICU) [5].
It has been determined that CFR gene (chloramphenicol-florfenicol resistance) codifies the 23s methyltransferase rRNA that confers resistance to linezolid. When evaluating this resistance, methicillin-resistant Staphylococcus aureus (MRSA) was found, which was also resistant to linezolid by the same mechanism [6]. Linezolid resistance in Enterococcus faecalis was also evaluated by mutation of the rRNA 23s gene. Resistance levels increase with the number of mutated copies of the gene and with the duration of exposure, so it was determined that the antibiotic dose seems to be critic in the dynamic and molecular basis of the resistance [7].
In Colombia, antimicrobial resistance surveillance was designed by the Colombian Integrated Program for Antimicrobial Resistance Surveillance (Coipars), who contributed to the execution of a study in the country with meat from 1003 poultry for retail sale in 23 departments of Colombia. A total of 556 enterococci were isolated between 2011 and 2012 in which the evidence showed a reduced susceptibility to linezolid due to the optrA gene in Enterococcus faecalis. This was the first time that the optrA gene was found in the Americans, the first time it was isolated was in China and later in Malaysia and Europe [8, 9].
The aim of this study is to describe the prescription of linezolid in a high complexity hospital in Bogotá, Colombia, considering the follow-up of the recommendations for the use of the drug included in clinical practice guidelines (CPG) of the hospital and its indications.
METHODS
A descriptive observational study of longitudinal section was performed with retrospective collection of the information of all patients who were prescribed linezolid during their hospitalization in the period from January 1st, 2017 to December 31st, 2018. Information on the diagnosis, medical history, indication, dose and the prescribing service of the drug were extracted from the medical history and were recorded in a collection form designed by the researchers. Quantitative variables are presented with measures of central tendency and dispersion. The analyses were performed in a Microsoft® Excel® 2016 spreadsheet.
RESULTS
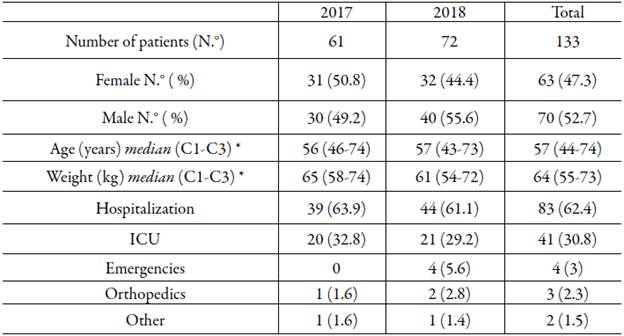
Information was collected from 133 patients treated during the study period. 31 women and 30 men were treated in 2017 and 32 women and 40 men in 2018. The sociodemographic characteristics and the service where the care was provided to the patients according to the year and the total attention are presented in table 1.
Of the total of patients, 21.8 % (29/133) had a history of kidney disease, 16.5 % (22/133) diabetes mellitus, 10.5 % (14/133) malnutrition, 9.7 % (13/133) edema and 4.5 % (6/133) some type of cancer.
The most frequent infections were those acquired in the community with 62.41 % (83/133), infections associated with health care occurred in 37.59 % (50/133) and were divided into nosocomial and those related to patient care.
The most frequently diagnosed infections during the study period were sepsis of different origin (pulmonary abdominal, urinary) with 22.6 % (30/133), followed by skin and soft tissue infection 16.5 % (22/133), the pneumonia with 12.8 % (17/133) and urinary tract infections with 9.8 % (13/133). Other infectious diagnoses with more than one case are presented in table 2. The other category corresponds to diagnoses with only one case or that could not be classified as such (for example: "other pleura conditions", lower limb thrombophlebitis, etc.).
Table 2 Infectious diagnoses of the patients followed.
| Infectious diagnoses | N.° | % |
|---|---|---|
| Sepsis | 30 | 22.6 |
| Soft tissue infection | 22 | 16.5 |
| Pneumonia | 14 | 10.5 |
| Urinary tract infection | 13 | 9.8 |
| Septic shock | 8 | 6,0 |
| Bacteremia | 4 | 3.0 |
| Cholangitis | 4 | 3.0 |
| Lung origin infection | 3 | 2.3 |
| Peritonitis | 3 | 2.3 |
| Tuberculosis | 3 | 2.3 |
| Abscesses | 2 | 1.5 |
| Lower limb cellulite | 2 | 1.5 |
| Osteomyelitis | 2 | 1.5 |
| Others | 23 | 17.3 |
| Total | 133 | 100.0 |
The prescription of linezolid was made mainly by professionals specialized in internal medicine with 73.7 % (91/133), followed by surgeons with 12 % (16/133) and infectious disease specialist with 7.5 % (10/133). Other data of interest is found in figure 1. Of the total of patients and independently of the prescriber, 76.7 % were referred to the infectious diseases service (102/133).
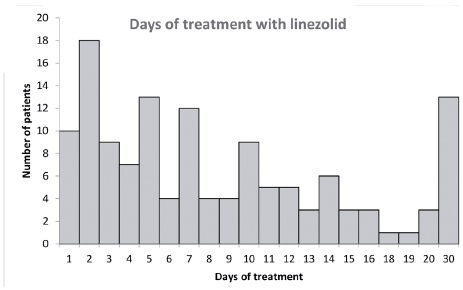
The days of treatment with linezolid were between 1 and 30 days, with a median of 10 days. The most frequent treatment time was 2 days with 13.5 % (18/133), followed by 5 and 30 days with the same frequency, which is 9.7 % (13/133) and 7-day treatments with 9 % (12/133). Figure 2 shows us in detail the treatment time.
It was identified that 69.9 % (93/133) underwent an antibiogram of which resistance to previous antibiotics occurred in 53.7 % (50/93) of the cases. There were no reports of resistance to linezolid.
Some microorganisms before or during the treatment with linezolid was identified in 81.2 % (108/133) of the patients, where it was found that the two most frequently identified microorganisms were Enterococcus faecium with 19.4 % (21/108) and Escherichia coli with 11.1 % (12/108). Other isolated bacteria were methicillin-resistant Staphylococcus aureus 9.2 % (10/108), Staphylococcus spp (epidermidis, aureus, haemolyticus, hominis, capitis) with 13.9 % (15/108) and others to a lesser extent. There were identified three cases where Candida albicans was recognized as a single microorganism.
In 33 % (44/133) of the patients, linezolid was used empirically (without using a previous antibiotic), while in 40.6 % (54/133) it was used as a second option (a previous antibiotic scheme). It was used as a third option in 20.3 % (27/133). Finally, in 6 % (8/133) of the patients, linezolid was prescribed after three previous antibiotics. The antibiotics most frequently used before prescribing linezolid were vancomycin with 44.9 % (40/89), piperacillin/tazobactam with 21.3 % (19/89) and meropenem with 12.3 % (11/89). Other antibiotics used prior to linezolid were daptomycin, tigecycline, ampicillin + sulbactam, ciprofloxacin, clarithromycin, oxacillin, amikacin, clindamy-cin and cefepime.
The reasons for using linezolid in the second or third line were the lack of response (described as worsening of the clinical picture) with 24.6 % (15/61), followed by other reasons among which resistant microorganisms are with 14.6 % (9/61) and adverse reactions to medications with 13.1 % (8/61).
An adequate response to linezolid was obtained in 61.6 % (82/133) of the patients, which was documented in the medical history as: clinical improvement with 46.3 % (38/82), microbiological cure 34.1 % (28/82) and discharged 19.5 % (16/82). The therapeutic objectives were not achieved in 37.7 % (51/133) of the patients. These cases were documented in the medical history as worsening of the infectious process (septic shock) in 62.7 % (32/51) and staggering to another antibiotic in 37.3 % (19/51).
DISCUSSION
According to the WHO, strategies to control bacterial resistance should be established through government guidelines, determining as a priority, creating or modifying national policies to prevent the incorrect use of antibiotics, be this product of the lack of knowledge of the community that has access to consumption of these drugs or of the excessive prescription without following the appropriate guidelines for the treatment of different pathologies [10].
Infectious diagnoses
Most of the indications for which linezolid was used correspond to what is authorized by the regulatory agency (Invima), such as pneumonia, sepsis and soft tissue infection, among others. Some diagnoses identified in the medical history as "viral pneumonia" and "systemic candidiasis" without another diagnosis are striking. It is possible that the clinical manifestations of these infections are similar to their bacterial analogues, and due to the severity of the clinical picture it was necessary to start with empirical therapy while identifying the microorganism. Another finding that is interesting to highlight is its indication in the treatment of urinary tract infection where there is a higher prevalence of gram-negative bacteria [11]. Considering the approved indications and recommendations of the CPG in the present study, cases were found compatible with inappropriate use, related to incorrect choice of the antibiotic. However, because the diagnosis was not correlated with the microorganism or by incomplete information in the medical history, it is not possible to conclude on its proper use.
Findings very similar to those obtained in the present study have been described in other studies carried out in Spain where in one of them nosocomial pneumonia is identified as the most frequent indication, followed by sepsis [12], and in another to skin and soft tissues infections [13]. These differences could happen due to the level of complexity and the fact that some services that the present study did include were excluded.
Prescribing professional
In this study, the specialist in internal medicine and the surgeon were identified as the main prescribers of linezolid. It should be taken into account that a high percentage was referred to consultation with the infectious disease specialist, and despite being in charge of supervising antimicrobial therapy, it is not necessarily the one who prescribes.
The role of prescriber in the spectrum of antibiotics determines that specific knowledge and training in clinical interpretation is required. Therefore, documents with linezolid in intensive therapy showed that this antibiotic was properly formulated or justified in up to 97 % of the cases, assuming that the work of a focus group with specialized knowledge had an impact on adequate antimicrobial use [14].
However, medication administration groups have been generated, which in the case of antibiotics is called "antimicrobial stewardship", models in which a medical specialist is not required but rather training. This is corroborated by the study carried out in 2014 in Caen in an institution similar to where the present study was conducted, documenting that in the period evaluated from 2009 to 2012 the formulation of linezolid was increased and only 60 % was considered properly indicated. After implementing practitioners' education, pre-authorization prior to dispensing medication from the pharmacy and the intervention of trained physicians to evaluate the inappropriate formulation, the formulation ratio decreased from 41 % in 2011 to 30 % in 2013. Although in 2009 the proper formulation ratio was 70 % and in 2013 60 %, the inappropriate formulation of this last period was modified in 62 % or suspended in 38 % with the advice of a trained physician (15). It is noteworthy that the institution where the study was conducted implemented the program of monitoring and careful use of antibiotics starting from 2017.
Treatment time
It is interesting to note that in almost a third of the treatments were performed for 1-3 days which is insufficient to treat any infection. This could mean that a good part of the treatments is started empirically and after identifying the microorganism it is de-escalate. In the previously related study, the median of treatment days was lower for a couple of days than that of the present study [13]. This high proportion of treatments of such a short duration could mean an inappropriate use of linezolid, possibly caused by and empiric use related to the clinical status of the patient or the absence of the infectious disease specialist for a timely interconsultation.
Treatment option
Although linezolid is not a first line antibiotic, under some circumstances it is considered that it can be used empirically as in soft tissue infection and pneumonia, especially when first line therapy is contraindicated (hypersensitivity, renal failure) [14-17].
In the patients followed, linezolid was prescribed in a group of patients that were not assessed by the infectious disease specialist and was used as a first line antibiotic, showing that a relevant percentage of patients received prescriptions not in accordance with the CPG that recommend linezolid as a last line antibiotic [17]. The lack of staging in the antimicrobial treatment is reflected in the high use prior to vancomycin linezolid.
About a third of the prescriptions were made empirically, moving away from the recommendations of the CPG that suggest the identification of the microorganism involved in the infection and the determination of its sensitivity or resistance before prescribing the linezolid [17, 18]. While the third part of the treatments in the present study used linezolid empirically; in the reference studies this practice is between 46.6 % and 76 % [12, 13]. These discrepancies may be due to the design of the studies (study period and inclusion and exclusion criteria) as well as the quality of the information in the medical histories, among other arguments.
Microorganism identified
Taking into account that linezolid has activity against gram-positive microorganisms, including methicillin-resistant staphylococci, penicillin-resistant pneumococci, vancomycin-resistant E. faecalis and E. faecium, the use in infections caused by these bacteria is successful. Similar results were described in the reference study [13]. This antibiotic has no activity against gram-negative (as is the case of urinary tract infections), so, having been used in infections caused by this group of microorganisms, the effectiveness of therapy in these patients is questioned [2, 18-20].
Therapeutic results
The conditions of potentially inappropriate use of linezolid found in this study could be considered as causes related to the non-achievement of the expected therapeutic objective, however, it should be clarified that the success of any pharmacological therapy depends on additional factors especially related to the clinical condition of the patient and that were not analyzed in this paper [21-24]. Comorbidities such as diabetes, renal failure, and the presentation of some signs such as the appearance of edema, may make it difficult to obtain successful therapeutic results or with optimal clinical results, because in these situations the pharmacokinetics of the antibiotic could be modified [21]. Likewise, the result of the therapy can be modified by the delayed bacterial growth associated with the development and detachment of biofilms on the surface of the devices used in the invasive approach during hospitalization [22, 23]. Both situations were described in some of the patients included in the analysis, finding a high proportion of use of devices such as catheters, probes or orotracheal tubes (97 % of patients).
The limitations of this study are related to the analysis of the medical records of the patients included, since in particular cases the information contained therein does not allow to generate a complete overview of the patient care process and the reasons that motivate therapeutic decision making by the professionals in charge of care.
It is recommended to optimize the diagnosis and treatment of infections in which antibiotics such as linezolid may be prescribed, in order to promote the proper use of antimicrobial agents. Some points to consider in the reasoned prescription process of these antibiotics include confirmation by antibiogram of susceptible strains, assessment by the infectious disease specialist and monitoring of CPG and management algorithms for particular clinical conditions. Although reasoned prescribing and evidence-based medicine consider the published literature on a topic, it is also important to have the prescriber's own experience and the patient's condition [25].
The regulatory entities in charge of pharmacovigilance in Colombia, led by the Ministry of Social Protection, supported by professional associations and WHO, are responsible for creating interdisciplinary groups that promote education in the community in general, health professionals and people that are involved in the creation, dispensation and administration of antibiotics, emphasizing the promotion and prevention of infections. These proposals should be supported by research laboratories that have the workforce, necessary instrumentation and quality standards to continue the research required to mitigate bacterial resistance and be constantly updated. In addition, it should have the support of the respective committees to carry out the correct follow-up of infections that require greater attention due to antibiotic resistance [10].
CONCLUSION
The present study shows that there is little adherence to the institutional CPGs in relation to the treatment time, the microorganism identification, and the use as first option. The absence of a full-time infectious disease specialist, the high workload and the continuous rotation of prescribing staff may be the cause of these results. Some cases of inappropriate use may be related to the clinical condition of the patient which requires empirical treatments.