INTRODUCTION
Polygonum acre H. B. K. (syn. Polygonum punctatum Elliot and Persicaria punctata Elliott; Polygonaceae) is popularly known as smart weed, water pepper, chilillo, catay dulce or erva de bicho [1]. The species belongs to the genus Polygonum, which has about 300 species that are widely distributed throughout the world [2]. This genus has been used in herbal medicines as infusions, decoctions, tinctures, and fresh juice, and in pharmaceutical preparations (creams, suppository, pills) due to a range of biological properties, including astringent, anti-inflammatory and antioxidant [3]. Studies have shown that species of this genus, such as P. hydropiperoides Michx., P. persicaria L. and P. acuminatum Kunth, have been used internally against diarrhea and intestinal parasites and externally for the treatment of hemorrhoids and non-rheumatic pain [2]. Similarly, different extracts of P. acre H.B.K. have exhibited anti-inflammatory [4], antidiarrheal [5], antihemorrhagic [6] and antimicrobial [5] activities, as well as a reductive effect on histamine-induced vascular permeability [1]. The use of this genus by indigenous people has been described in Argentina and the French Guiana. Indigenous peoples of Guiana successfully use a gel prepared with sap of P. acuminatum H.B.K. to treat eye inflammation [7]. An infusion or fresh juice made with the aerial parts of P. acre is widely used by the Indians of Toba (northern Argentina) as a disinfectant for wounds and skin rashes [8].
Pharmacognosy studies of this genus reveal a great variety of secondary metabolites, such as phenolic compounds (flavonoids and tannins) [9], sesquiterpenoids (polygodial) [10] and others [11]. Terpenoids, such as polygodial and b-bisabolene, a major component of the essential oil, have been related to the antifungal, anti-inflammatory and antibacterial activities compatible with the traditional use [12]. Flavonoids are considered the most common components found in members of the genus Polygonum and have previously been used as chemotaxonomic markers of the genus, playing an important role in the systematics of Polygonaceae species [13]. In addition, plants with high concentrations of polyphenols, such as flavonoids and tannins, are of interest owing to their antioxidant properties [14]. Although the literature on phenolic compounds and antioxidant activities of several species of Polygonum is available, little is known about P. acre.
The aim of this study was to characterize the chemical profile of P. acre extracts obtained by infusion, decoction, tinctures, aqueous and ethanolic extraction and to investigate their antioxidant activities using in vitro assays. This study could emphasize the ethno-pharmacological relevance of the Polygonum genus by reinforcing the scientific basis for its use in traditional medicine.
METHODOLOGY
Chemicals and reagents
ABTS (2,2'-azino-bis) (3-ethylbenzothiazoline-6-sulfonic acid), BHT (butylated hydroxytoluene), phenolic standards (gallic acid, rutin, and quercetin), and TROLOX (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were obtained from Sig-ma-Aldrich (St. Louis, MO, USA). Reduced nicotinamide adenine dinucleotide (NADH), nitroblue tetrazolium (NBT), and 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) were obtained from Amresco (Dallas, TX, USA). Methanol-grade HPLC was obtained from JT Baker (Xalostoc, Mexico). Water was purified using an ultra-purifier MS 2000 model from Gehaka (Sao Paulo, SP, Brazil). Folin-Ciocalteu reagent was prepared from Chromate (Sao Paulo, SP, Brazil). All other reagents were of analytical grade.
Preparation of plant extracts from the aerial parts of P. acre
Traditional extractions
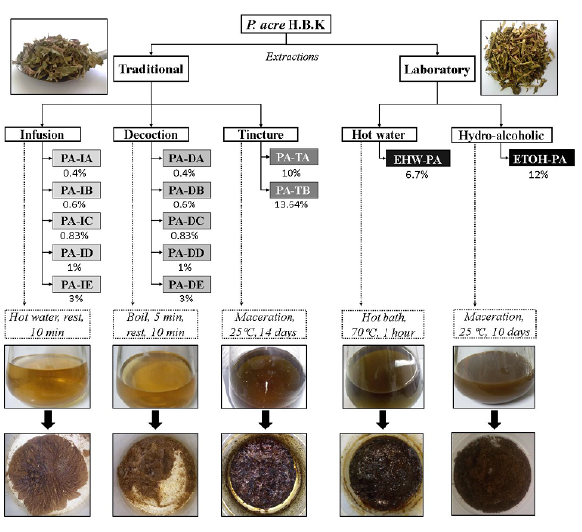
Traditional extractions were carried out using commercially available dry aerial parts of P. acre H. B. K. (Chá & Cia Ervas Medicinais, São José dos Campos, Brazil) with the certificate analysis (# 43) from Quimer Comercial Ltda (São Paulo, Brazil) based on the National System of Genetic Resource Management and the Associated Traditional Knowledge, SISGEN, (AE95785). The process used the following standardization: 1 tablespoon corresponding to 2 g of dry material and 1 teacup corresponding to 240 mL. Infusions were made with different concentrations, 0.4, 0.6, 0.83, 1.0 and 3.0% (w/v), identified by the letters A (as recommended by Chá & Cia Ervas Medicinais), B [15], C [16], D [17], and E [18], respectively, as illustrated in Figure 1. Tinctures were prepared by hydroethanolic maceration (70% v/v, 1L), at two concentrations A 10% (w/v) [15,16] and B 13.64% (w/v) [15] at room temperature (25°C) for 14 days. After the extraction procedure for infusion, decoction, or tincture, the content was filtered using a vacuum system. The filtered material was then concentrated in a rotary evaporator (801 Fisatom, São Paulo, Brazil), frozen, and freeze-dried, yielding the extracts illustrated in Figure 1.
Laboratory extractions
The laboratory extractions were performed by following the protocols adapted from HIT [16] to compare their yields and extracts' phytochemical profiles with those obtained from traditional methods. We carried out an aqueous extraction (6.7% m/v, 1.5 L) in a water-bath (70 °C). The extraction was carried out for 1 h with constant mechanical stirring. The extract was filtered under vacuum, concentrated in a rotary evaporator under reduced pressure at 40°C, freeze-dried, and designated as EHW-PA. The second extraction was made by hydroethanolic maceration (70% v/v) performed at room temperature (25°C) for 10 days. The flask was kept protected from light. After the extraction, the material was filtered under vacuum, concentrated at same conditions mentioned before, and freeze-dried, yielding the extract called ETOH-PA (Figure 1). Both, EHW-PA and EOTH-PA, were stored at -4°C in the dark.
PA-IA to IE - extract obtained by infusion of aerial parts of P. acre at indicated concentrations; PA-DA to DE - extract obtained by decoction of aerial parts of P. acre at indicated concentrations; PA-TA and PA-TB - extract obtained by tincture of aerial parts of P. acre at 10% and 13.64%, respectively; EHW-PA - extract of aerial parts of P. acre at 6.7% (w/v) obtained by aqueous extraction at 70 °C; ETOH-PA - extract of aerial parts of P. acre at 12% (w/v) obtained by hydroethanolic maceration (70% v/v) at 25 °C for 10 days.
Chemical characterization
Colorimetric determination of total phenolic, flavonoid, condensed, and hydrolyzed tannin contents
Samples of infusion, decoction, tinctures, and laboratory extracts were used to determine the total phenolic content using the Folin-Ciocalteu reagent (FCR) microassay adapted from the method of Singleton & Rossi Jr [19], utilizing gallic acid as standard (R2 = 0.995). The content of flavonoids was measured by aluminum chloride complexation described by Woisky & Salatino [20] and the results were compared with a rutin standard curve (R2 = 0.999). The condensed tannins (CT, or proanthocyanidins) were measured using an adaptation of the vanillin method described by Queiroz et al. [21] using a standard curve of epicatechin (R2 = 0.997). Hydrolysable tannins were analyzed with potassium iodate in a microassay adapted from Willis & Allen [22] utilizing a tannic acid calibration curve (R2 = 0.990). All tested samples were read on a microplate reader (EPOCH model, BioTek, Winooski - USA), using 96-well flat bottom microplates (Techno Plastic products, TPP AG, Trasadingen, CH).
High-performance liquid chromatography analysis (HPLC)
The phenolic analysis was conducted using an Agilent 1200 Series high-performance liquid chromatography (HPLC) system (Agilent Co., Santa Clara, USA) equipped with a vacuum degasser (G1322A), quaternary pump (G1311A), manual injector (Rheodyne, 7725i), and a multi-UV-VIS wavelength detector (G1365D) operating at a wavelength of 254, 280, 300, 325, and 375 nm, using an Agilent Eclipse XDB-C-18 column (150 mm x 4.6 mm, 5 [im particle size). The mobile phase used was the following elution gradient with acetonitrile: 5 % (0 - 5 min), 10 % (5 - 10 min), 30 % (10 - 20 min), 50 % (20 - 30 min) and 5 % (30-35 min) at a flow rate of 1 mL/min. Twenty microliters of the samples were injected through a manual injector. EHW-PA was diluted in methanol: water (1:9 v/v) at a concentration of 5 mg/mL and filtered through a 0.22-µm membrane filter (JetBiofil, Guangzhou, China) prior to injection. The standards (gallic acid, chlorogenic acid, quercetin, and rutin) were prepared individually in methanol at a 100 µg/mL concentration. The phenolic compounds were identified by comparing their retention times with standards. The EZChrom Elite program via Windows 7 was used for system control and data analysis.
In vitro antioxidant assays
ABTS radical scavenging assay
The antioxidant assay, using the ABTS radical (ABTS●+) was performed using a microassay adapted from the method previously described by Re et al. [23]. Initially, we prepared a stock solution of partially oxidized ABTS cations by adding 88 µL of potassium persulfate solution (2.45 mM, ultra-purified water) to 5 mL of an ABTS solution (7 mM, ultra-purified water), which was then left to react for 16 h at room temperature and protected from light. For the test, we diluted 1 mL of the ABTS●+ stock solution in phosphate buffer (75 mM, pH 7.4) and corrected the absorbance with ultra-purified water or with the radical solution to reach 0.7 ± 0.02 at 734 nm. In a 96-well plate, we pipetted 20 µL of the sample of P. acre extracts or commercial standards (gallic acid, rutin, and BHT) (3.9 - 500 µg/mL), or TROLOX (12.5 to 200 µM), or phosphate buffer (blank) solutions and 220 µL of the ABTS●+ solution (diluted and adjusted). The plate was allowed to rest for 6 min while being protected from light; the absorbance of reaction mixture with ABTS●+ was measured at 734 nm, on a microplate reader (EPOCH model, BioTek, Winooski, USA). We used a linear regression (R2 = 0.995) to express the antioxidant activity in equivalence to TROLOX. The percentage of ABTS●+ scavenging was calculated and expressed as mean ± standard deviation (SD) by the formula: Antioxidant activity (%) = [(Ac - A)/Ac] x 100, where Ac and A are the control and samples average absorbance, respectively.
Ferric reducing antioxidant power (FRAP) assay
The iron reduction power of the extracts was investigated using the FRAP method [24]. At low pH, TPTZ forms a complex of a yellow rust color with reduced iron [Fe(III)(TPTZ)2]3+. Upon the addition of an antioxidant, the Fe(III) to Fe(II) reduction occurs, changing the color of the reaction medium to a dark blue. The assay was adapted from the method described by Müller et al. [25] and consisted of adding 20 of the sample of P acre extracts or commercial standards (gallic acid, rutin, and BHT) (3.9 - 500 µg/mL), or ferrous sulfate (10 to 700 µM) or ultra-purified water (blank), plus 30 of ultra-purified water and 200 of FRAP reagent. The plates were incubated at 37 °C for 8 min and then read for absorbance at 595 nm, on a microplate reader. The FRAP solution consisted of 10 parts of potassium acetate buffer (0.3 M, pH 3.6), 1 part of ferric chloride (20 mM) and 1 part of the TPTZ solution (10 mM in HCl, 40 mM). The FRAP values were calculated by applying linear regression on the standard curve (FeSO4-7H2O, 50 to 700 µM) and estimating concentrations in equivalents of Fe2+ per mg of dry extract (R2 = 0.996).
Superoxide anion radical scavenging activities
The scavenging capacity of the superoxide radical (O2 ●-) was determined with a microassay adapted from Nishikimi et al. [26] and Gomes et al. [27] This method tested the molecule's capacity to decrease the extent of NBT (nitro blue tetrazolium) reduction after scavenging the O2»-, generated by the nicotinamide adenine dinucleotide and phenazine methosulfate system (NADH-PMS) in the reaction medium. Therefore, lower NBT reduction led to lower absorbance at 560 nm. The percentage of O2 ●- scavenging (expressed as mean ± SD) was obtained from the equation: O 2 ● -radical scavenge (%) = [(Ac - A)/Ac)] x 100, where Ac and A stand for the mean absorbance of the control and the sample solution, respectively. Briefly, we added 100 of the sample of P acre extracts or commercial standards (gallic acid, and rutin) (3.9 500 μg/mL), or phosphate buffer solution (19 mM, pH 7.4) (blank), 50 μL of NBT (645 μM in phosphate buffer 19 mM, pH 7.4) and 50 μL of PMS (16.2 μM in phos phate buffer 19 mM, pH 7.4). After mild stirring, we added 100 μL of NADH (498 μM in phosphate buffer 19 mM, pH 7.4) and the plate was incubated, protected from the light, at room temperature for 5 min, followed by reading absorbance at 560 nm.
Statistical Analysis
Results were calculated as mean ± SD (n = 3). The results of the colorimetric determinations and antioxidant tests were analyzed by one-way analysis of variance (ANOVA), followed by Tukey's multiple comparison tests, using the GraphPad Prism 5 software. The differences were considered statistically significant at P ≤ 0.05. The half maximal effective concentration (EC50) corresponded to the concentration of the extract that was able to capture 50% of the free radicals; it was calculated using linear equations (R2 ≥ 0.95) obtained by linearizing the activity curves. Pearson's correlation was used to analyze the relationship of the total phenolic or flavonoid content with the antioxidant activity. For the analysis of correlation coefficient values (r, Pearson's correlation coefficient), we considered the following values for correlation categorization, high: from 0.7 to 1, moderate: 0.5 to 0.7, low: 0.3 to 0.5, and insignificant: less than 0.3 [28].
RESULTS AND DISCUSSION
Chemical characterization of extracts obtained by traditional and laboratory protocols from aerial parts of P. acre
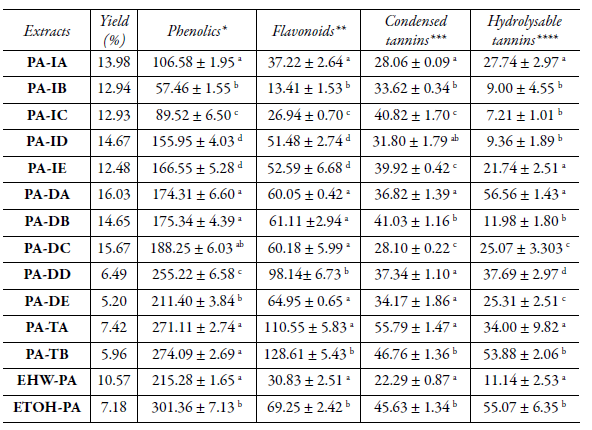
The chemical characterization data of total phenolic, flavonoids, condensed tannins, and hydrolysable tannins of P. acre extracts are presented in Table 1. Regarding phenolic contents of traditional extractions, the tinctures (PA-TA and PA-TB) had a higher average content of total phenolic (232.60 mg of gallic acid equivalent (GAE)/g dry extract), than the infusions (115.21 mg; PA-IA, PA-IB, PA-IC, PA-ID, and PA-IE) and decoctions (197.78 mg; PA-DA, PA-DB, PA-DC, PA-DD, and PA-IE) (Table 1). The higher total phenol content observed in the tinctures (obtained by hydroethanolic maceration) corroborated with the literature data, since phenolic compounds are regularly more soluble in organic solvents less polar than water [29, 30]. It was observed that the total phenol content was statistically different between the different extracts obtained by infusion or decoction compared to that between the extracts obtained by the same protocol. The ETOH-PA obtained by hydroethanolic extraction (12% w/v, at 25 °C for 10 days) presented the highest phenolic content (301.36 ± 7.13 mg of GAE/g dry extract) (Table 1). For the EHW-PA, the total phenol content was 215.28 ± 1.65 mg of GAE/g dry extract (Table 1).
The phenolic compounds occurring in all extracts were mostly flavonoids (Table 1). The tinctures (PA-TA and PA-TB) displayed the highest contents of these compounds, corresponding to 110.55 and 128.61 mg of rutin equivalent (RE)/g dry extract, respectively. All tested extracts presented condensed tannin content in the ranges of 22.29 to 55.79 mg of epicatechin equivalent (EPE)/g dry extract (Table 1). For hydrolysable tannins, the values varied between 0.74 and 50.50 mg of tannic acid equivalent (TAE) /g dry extract (Table 1).
All indicated percentage yields were calculated with respect to the plant initial dry mass. *Determined according to the method by Singleton & Rossi Jr [19], expressed as mg GAE/g of dry extract. ** Determined according to the method by Woiski & Salatino [20], expressed as mg RE/g of dry extract. *** Determined according to the method by Queiroz et al. [21], expressed as mg EPE/g of dry extract. ****Determined according to the method by Willis & Allen [22], expressed as mg TAE/g of dry extract. Different letters in each column represent significant differences between the samples by the Tukey test (p < 0.05). PA-IA, PA-IB, PA-IC, PA-ID, PA-IE - extract obtained by infusion of aerial parts of P acre at 0.4, 0.6, 0.83, 1, 3% (w/v), respectively; PA-DA, PA-DB, PA-DC, PA-DD, PA-DE - extract obtained by decoction of aerial parts of P. acre at 0.4, 0.6, 0.83, 1, 3% (w/v), respectively; PA-TA and PA-TB - extract obtained by tincture of aerial parts of P. acre at 10 and 13.64% (w/v), respectively; EHW-PA -extract of aerial parts of P. acre at 6.7% (w/v) obtained by aqueous extraction at 70 °C; ETOH-PA - extract of aerial parts of P. acre at 12% (w/v) obtained by hydroethanolic maceration (70% v/v) at 25 °C for 10 days.
The phenolic content values of the present study were higher than those reported by Lima et al. [31] (1.57%, crude material of leaves) for the aerial parts of P. acre H.B.K var. aquatile Meisn. The hydromethanolic extracts (80% v/v) of aerial parts of P. capitatum, P. chinensis, P. cuspidatum, and P. multiflorum presented a total phenolic content lower than recorded for P. acre of 86.9, 441.5, 63.3, and 12.7 mg (of GAE/g dry extract), respectively [32]. The hydroethanolic extraction (50% v/v) from the whole P. aviculare plant was around 677 mg of GAE/g dry extract [33]. The hydroethanolic extraction (80% v/v, 50 °C, 2 h) from the aerial parts of P minus showed a statistically higher content than the aqueous extracts of the same plant [34]. This later finding is similar to our observation for P. acre. The flavonoid content showed for PA-TA, PA-TB and EOTH-PA were higher than those reported for hydromethanolic extracts of other Poligonum species [32, 33] (P multiflorum, P. chinensis, P. capitatum, and P. cuspidatum).
EHW-PA was selected for HPLC analysis as it showed a higher yield (10.58 % w/m) and a phenolic content > 200 mg GAE / g dry extract. The preliminary phenolic analysis profile of EHW-PA showed a chromatogram with 20 peaks (data not shown). Although, it was possible to verify the presence of gallic acid, rutin, and quercetin through comparisons with the retention times of standard compounds, under the conditions tested. Other studies also described these compounds in the HPLC analysis of the phenolic content in hydromethanolic extracts of the aerial parts of species of the genus Polygonum (P. lapathifolium, P. divaricatum, P. angustifolium, P. amphibium e P. aviculare) [9,35].
In vitro antioxidant activity
Figure 2 shows the response curves representing the percentage of radical scavenging activity versus concentration (3.9 to 500 µg/mL) of P. acre extracts. For comparison purpose, one extract with higher content of phenolic compounds of each form of use, i.e., infusion (PA-IE), decoction (PA-DD) and tincture (PA-TB), and those obtained by laboratory extraction (EHW-PA and ETOH-PA), and commercial phenolic standards (gallic acid, rutin, and BHT) were selected for the antioxidant activity assays.
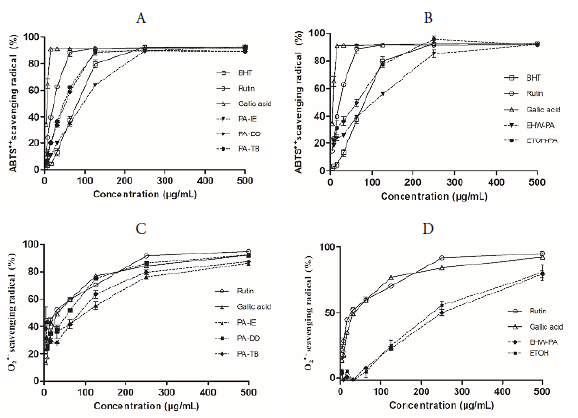
Figure 2 Profile of the activity curves of ABTS●+ (A, B) and O2 ●- scavenging properties (C, D) of PA-IE, PA-DD, PA-TB, EHW-PA, and ETOH-PA obtained from the aerial parts of Polygonum acre and commercial standards (BHT, rutin, and gallic acid).
The ABTS assay has been the most widely employed method for estimation of antioxidant activity and is based on electron transfer [34, 35]. All tested extracts and standards showed a concentration-dependent activity of ABTS●+ in the capture assay, remaining at around 90% of the activity at the highest concentration (Figure 2). Regarding laboratory extractions, the ETOH-PA was the one that exhibited the best results (Figure 2 and Table 2). The traditional extractions displayed the following sequence of activity: PA-TB = PA-DD > PA-IE, where the tincture and the decoction presented similar results as indicated in Table 2.
Table 2 Antioxidant activity of the extracts obtained from the aerial parts of Polygonum acre.
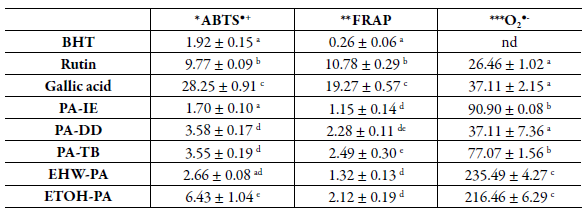
* Determined in accordance with the method reported by Re et al. [23], expressed as mM equivalent of TROLOX per g dry extract. ** Determined in accordance with the method reported by Miller et al. [25], expressed as mM equivalent of Fe2+ per g dry extract. ***Determined in accordance with the method reported by Nishikimi et al. [26] and Gomes et al. [27] expressed as EC50 (µg/mL). Different letters in each column represent significant differences by the Tukey test (p < 0,05). PA-IE - extract obtained by infusion of aerial parts of P. acre at 3% (w/v); PA-DD - extract obtained by decoction of aerial parts of P acre at 1% (w/v); PA-TB - extract obtained by tincture of aerial parts of P acre at 13.64% (w/v); EHW-PA - extract of aerial parts of P. acre at 6.7% (w/v) obtained by aqueous extraction at 70 °C; ETOH-PA - extract of aerial parts of P. acre at 12% (w/v) obtained by hydroethanolic maceration (70% v/v) at 25 °C for 10 days.
PA-IE - extract obtained by infusion of aerial parts of P. acre at 3% (w/v); PA-DD -extract obtained by decoction of aerial parts of P. acre at 1% (w/v); PA-TB - extract obtained by tincture of aerial parts of P. acre at 13.64% (w/v); EHW-PA - extract of aerial parts of P acre at 6.7% (w/v) obtained by aqueous extraction at 70 °C ; ETOH-PA - extract of aerial parts of P. acre at 12% (w/v) obtained by hydroethanolic maceration (70% v/v) at 25 °C for 10 days.
A strong correlation (r = 0.9116) and a statistical difference (P = 0.0311) was observed between the content of phenolic compounds and the antioxidant activity by the ABTS*+ capture assay, in contrast to the correlation between the flavonoid content and antioxidant activity (r = 0.2734 and P = 0.6563) (Figure 3).
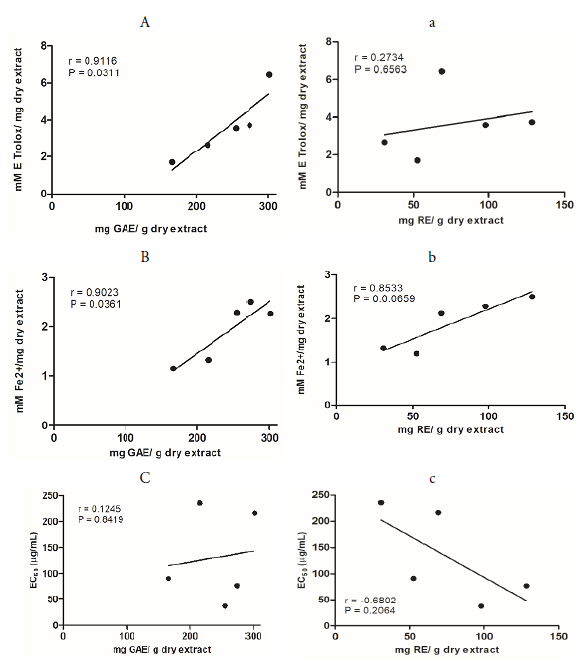
Figure 3 Correlation between ABTS*+ scavenging activity (A, a), ferric reducing antioxidant power (B, b), superoxide anion radical scavenging activity (C, c), and total phenolic (mg GAE/ g dry extract) or flavonoid (mg RE/ g dry extract) content of PA-IE, PA-DD, PA-TB, EHW-PA, and ETOH-PA obtained from the aerial parts of Polygonum acre. The variables were significantly correlated at p ≤ 0.05 (two-tailed).
The total antioxidant activity was confirmed by analyzing the reduction of the tripyridyltriazine ferric complex (Fe3+-TPTZ) to ferrous tripridyltriazine (Fe2+-TPTZ) [37]. Among all samples tested, the PA-TB showed the highest iron-reducing activity (Table 2). The rutin and gallic acid standards showed the highest activities of 10.78 and 19.97 mM Fe2+/g of dry extract, respectively. This test also showed a strong and significant correlation of antioxidant activity with the content of phenolic compounds (r = 0.9023) and P = 0.0361) and flavonoid content (r = 0.8979 and P = 0.0389) (Figure 3). In other studies, an extract obtained from ethyl acetate maceration of P. minus leaves with 227 mg GAE / g of dry extract showed an antioxidant activity at 2.435 mM Fe2+/mg of dry extract via the reducing power assay [38]. This result is quite consistent with that obtained for PA-TB, which presented about 274.09 mg GAE / g of dry extract and a reducing power of 2.49 mM Fe2+/mg of dry extract. Shahraki [39] found a similar value for the reducing power with the FRAP assay (2.11 mM Fe2+/mg of dry extract) on a methanolic extract obtained from the aerial parts of P patutum; however, the phenolic content of this sample was higher (415 mg GAE / g of dry extract).
P. acre extracts, along with the standards (gallic acid and rutin), were analyzed in terms of capture capacity of the O2 ●-. The standards presented a concentration-dependent activity as shown in Figure 2. At 500 µg/ml concentration, it was observed no statistical difference 92.19 and 95% of capture capacity of the O2 ●- for gallic acid and rutin, respectively. At the same concentration, only the decoction extract (PA-DD) had a similar result (92.31%). The results for PA-IE, PA-TB, EHW-PA and ETOH-PA were 86.11, 87.38, 79.35, and 83.57%, respectively. Despite the considerable antioxidant activity, no correlation between the total phenolic content (r = 0.1245, P = 0.8419) or flavonoid content (r = -0.6802, P = 0.2064) and the antioxidant activity was observed (Figure 3).
Other species of the Polygonum genus are known to have high superoxide anion scavenging power. The aqueous extract obtained from P. multiflorum leaves (100 mg/kg) presented antioxidant activity higher than 80% for the superoxide radical capture [40] and the antioxidant activity was related to the presence of emodin (anthraquinone) and quercetin. On the other hand, the methanolic extract of P. glabrum presented an EC50 around 36.98 µg/mL with the activity being related to the presence of a wide range of secondary metabolites, including phenolic compounds (3-hydroxy-5-meth-oxystilbene) and flavonoids, such as pinocembrim and pinocembrim-5-methyl ether [41]. The hydroethanolic extract of P. aviculare displayed an EC50 of 0.8 µg/mL, which is a low value when compared to the standard catechin (EC50 40 µg/mL) [42]. Several authors have reported that flavonoids can efficiently inactivate free radicals [43]. Moreover, Robak & Gryglewski [44] demonstrated that quercetin, myricitrin and rutin are also powerful O2 ●- inhibitors. According to the authors, the compounds were tested in their isolated forms and not as a mixture of compounds [44] that would be typically found in plant extracts. Therefore, it may be inferred that the activity of extracts is attributable to the sum of the effect of their constituents. Therefore, according to the data obtained in the present study, it is suggested that the absence of a correlation between the phenolic compound or flavonoid content and the antioxidant activity from the superoxide assay is due to the presence of antioxidative compounds that could not be identified. Additional experiments are required to prove this possibility.
CONCLUSIONS
Fourteen extracts of P. acre were obtained by using traditional and laboratory protocols. We have evaluated the chemical phenolic compositions and the antioxidant properties of these extracts. To our knowledge, this is the first time that a comparative study of the chemical characterization and antioxidative activity of extracts from P. acre, obtained by the form of the traditional use, has been described in the literature. The herbal medicines of the aerial parts of P. acre present a source of bioactive compounds with antioxidant properties. A positive correlation between the total phenolic content and the antioxidant activity was verified by ABTS●+ capture and FRAP assays. In contrast, a low to moderate correlation between the flavonoid content and antioxidant activity was observed. In conclusion, this study reinforces the ethnopharmacological relevance of the genus Polygonum, contributing to the scientific basis for the use of P. acre traditional preparations.