INTRODUCTION
The scientific studies aiming to isolate Cannabis-specific bioactives started in the second half of 19th century [1, 2], after the identification of different species [3, 4] and the publication of a detailed study on their possible risks and medicinal applications [5, 6]. At the beginning of the second half of the 20th century the member countries of the United Nations decided to include Cannabis derivatives in the list of the most dangerous narcotic substances, which are those that present harmful risks that do not compensate for possible therapeutic applications [6].
The specific bioactives were called phytocannabinoids, with cannabidiol (CBD) and Δ 9-tetrahydrocannabinol (Δ9-THC) being the main ones. These two substances are classified as structural isomers, as they have the same number of atoms of each element linked differently. The chemical structure of both was elucidated by Hydrogen Nuclear Magnetic Resonance (1H-NMR) analyses [7, 8]. The NMR techniques are based on a phenomenon that occurs with nuclei that have magnetic and angular moments and are analyzed by the resonant frequency (chemical displacement, S) with which the spin of the nucleus oscillates in the presence of a magnetic field, being this dependent on your neighborhood. The technique related to carbon nuclei (13C-NMR) are also commonly evaluated but require a longer analysis time due to less sensitivity [9].
The complete elucidation of the structure of these isomers allowed that in 1971 the A9-THC was included in the United Nations List of Psychotropic Substances, due to its potential to generate changes in brain functions such as perception, mood, behavior, and consciousness. Despite this, in the following years, studies demonstrated a significant antiemetic potential of this pure phytocannabinoid, thus making its synthetic version a common means to minimize the adverse effects of chemotherapy. At the same time, the absence of toxicity, side effects, and psychotropic effects of CBD, coupled with several promising medicinal results, has made it the most debated and analyzed phytocannabinoid in recent decades [6].
In 2018, the World Health Organization (WHO) did an indepth review of medical Cannabis and suggested to the United Nations Office on Drugs and Crime (UNODC) important updates on this topic [10]. The main suggestion was the recognition of the medicinal potential of Cannabis, to make the medical handling of derivatives less bureaucratic. The approval of the theme only managed to be carried out by most of the member countries of the United Nations at the end of 2020 [11]. In the review, WHO also highlighted that the three-dimensional structure of CBD obtained naturally may be different from that of synthetic CBD available on the market [10, 12]. Besides that the natural (-)-CBD enantiomer has a different action potential than (+)-CBD [10, 13].
Enantiomers can be identified using two-dimensional (2D) NMR techniques. The 2D-NMR techniques can analyze homonuclear (e.g.: 1H,1H) and heteronuclear (e.g.: 1H,13C) correlations and their data of scalar and spatial relationships allow to characterize important details of the chemical structure of a substance [9]. The 2D-NMR data for phytocannabinoids are not commonly described in scientific journals or have not yet been analyzed. Thus, this work aims to present experimental data of 1D-NMR and 2D-NMR, obtained from a sample of non-synthetic CBD with high purity. The detailed elucidation of the three-dimensional structure of this substance from unpublished NMR data allows it to be easily distinguished from its enantiomer and other isomers.
MATERIALS AND METHODS
Obtaining non-synthetic cannabidiol
The CBD used in this work was manufactured by Isodiol (Iso International, LLC -Batch Number: F191106), located in California - United States of America (USA), being registered internationally under the name ISO99tm - Bioactive Anhydrous Hemp OilTM. The product, of CBD purity ≥ 99%, is obtained from Industrial Hemp, which has the following definition in the country (2018 Farm Bill): "the plant Cannabis sativa L. and any part of such plant, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis". The research project for handling the product in Brazil was authorized by the Brazilian Health Surveillance Agency (ANVISA), under registration number AEP 005/2020. The authorization to import the product (AI-468-2020) could only be completed after receipt of a "Letter of no objection to export" issued by the Drug Enforcement Administration (DEA) because in Brazil the handling of the non-synthetic CBD requires greater control than for synthetic CBD.
NMR analysis
NMR analysis were conducted using a 500 MHz Nuclear Magnetic Resonance Spectrometer (Bruker, AVANCE III HD), at the Central Research Support Complex (COMCAP/UEM).
The analysis related to the hydrogen nuclei in 1D (1H) and 2D (1H,1H) were conducted from a sample with approximately 3.5 mg and 0.6 mL of deuterated chloroform (Sigma-Aldrich, 99.8%). Homonuclear Correlation Spectroscopy (COSY) was conducted to identify signs of proton coupling in twin (adjacent) or vicinal (two-away) carbons. The analysis of Nuclear Overhauser Effect Spectroscopy (NOESY) was also conducted, for correlating hydrogens that are spatially close.
The sample was subsequently concentrated (≅ 20 ~ 25 mg in 0.6 mL) for analyzes related to carbon nuclei in 1D (13C) and 2D (1H,13C). The multiplicity of substitution of carbon atoms was determined by the analysis of Distortionless Enhancement by Polarization Transfer with pulse angle of 150° (DEPT 135), which provided negative signals for CH2 and positive signals for CH and CH3. The spectrometric experiments of Heteronuclear Single-Quantum Correlation (HSQC), which present unique signals for protons linked to heteronuclei, and of Heteronuclear Multiple Bond Correlation (HMBC), which demonstrates the signals of protons close to distant heteronuclei by 2 or 3 bonds, also were conducted.
Data processing was conducted using the Mnova software (Mestrelab Research). In addition, the online software called GeoGebra 3D was used to analyze the three-dimensional geometry of the CBD previously described by X-ray diffraction.
RESULTS AND DISCUSSION
1D-NMR spectrum
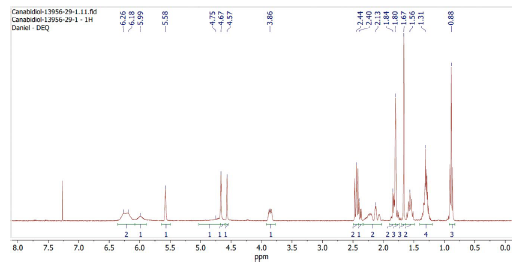
The 1H-NMR spectrum obtained is shown in Figure 1. The displacement S = 5.57 ppm was used as a reference for the integral equivalent to one proton. A total of 30 protons, as expected, was observed in the sample, with δ = 7.26 ppm referring to the solvent.
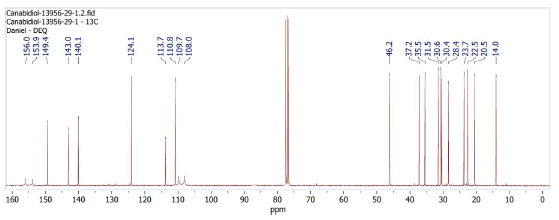
The 13C-NMR spectrum, shown in Figure 2, allows confirming the total of 21 carbons in the sample. The signs of displacement between 75 and 80 ppm are related to deuterated chloroform.
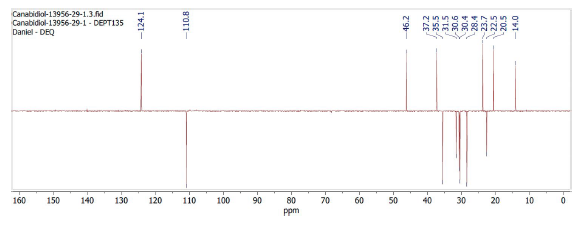
The DEPT 135 spectrum, shown in Figure 3, allows us to observe 7 carbons are CH2, by negative peaks, and that 6 carbons are CH or CH3, by positive peak. Therefore, the spectrum identifies only 13 of the 21 carbons in the sample.
2D-NMR spectrum
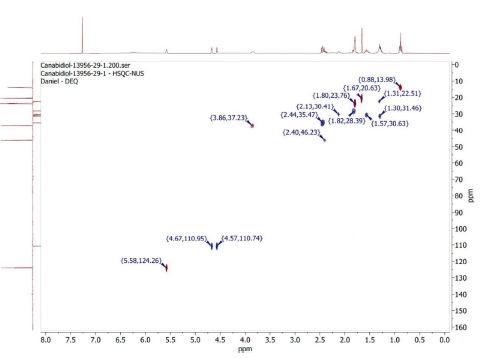
The HSQC spectrum, shown in Figure 4, allows identifying, in blue, which protons are related to the 7 CH2 groups in the sample. The spectrum also makes it possible to identify, in red, that there are 3 CH groups and 3 CH3 groups in the sample.
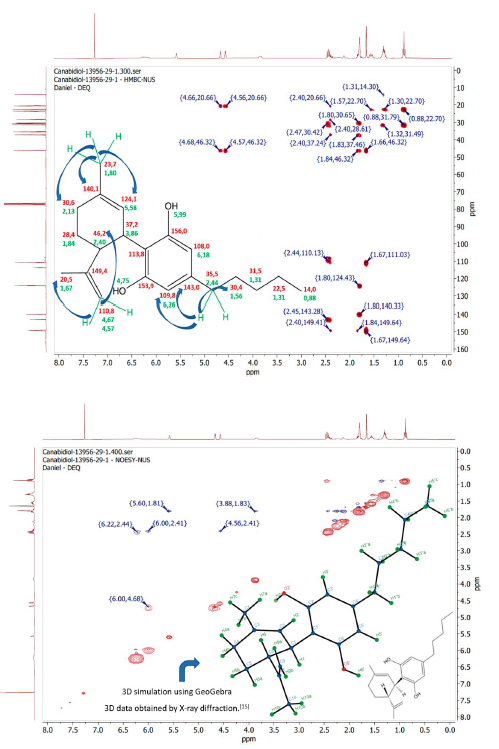
Figure 5 2D-NMR spectrum with scalar proton correlations of the (-)-CBD sample: a) HMBC (heteronuclear: 1H,13C); b) COSY (homonuclear: 1H,1H).
The HMBC (figure 5a) and COSY (figure 5b) spectrum, which demonstrate scalar interactions of protons with carbons and other protons, respectively, make it possible to identify the exact location of each atom in the plane structure of the CBD. In the COSY spectrum, only the upper diagonal was analyzed because it is related to homonuclear interactions. The spectrum allows observing that in total there are 5 CH groups in the molecule and not just the 3 shown by the HSQC spectrum.
The important characteristics of the three-dimensional structure of the (-)-CBD can be seen from the NOESY spectrum [14], shown in figure 6. The spectrum also demonstrates homonuclear interactions, so only one diagonal of the spectrum has been plotted. The data obtained were compared with the chemical structure previously elucidated by X-ray diffraction [15].
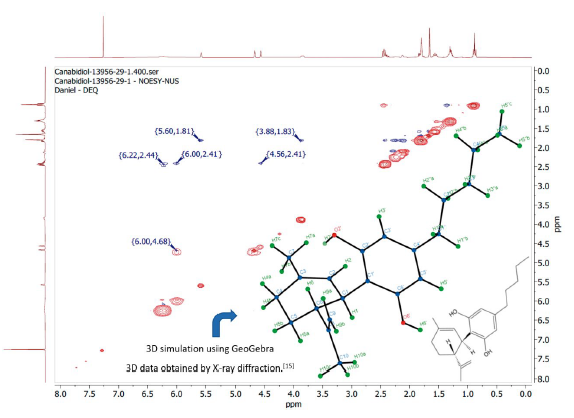
Figure 6 NOESY spectrum of the sample (-)-CBD compared to a simulated three-dimensional structure obtained from X-ray diffraction data described in the literature.
The spectrum shows that H2'OH, δ = 6.00 ppm, is close to H6 (2.04 Å) and H9a (2.92 Å ), δ = 2.41 ppm and δ = 4.68 ppm, respectively, thus proving that the H6 is really oriented upwards from the adopted plan. Therefore, it is possible to confirm that the (-)-CBD has the hydroxyl groups in opposite directions in space, as previously described by X-ray diffraction data [15], although there is another reference that contradicts this characteristic [16]. It is also possible to confirm that H1, δ = 3.88 ppm, is oriented downwards from the adopted plan because it is spatially close to H5 (2.28 Å), δ = 1.83 ppm. The distances mentioned were calculated using the online GeoGebra 3D software.
CONCLUSIONS
The 1D spectrum, 1H-NMR and 13C-NMR, showed chemical displacements equivalent to those previously described in the literature [17, 18] and are sufficient to differentiate the CBD and Δ9-THC isomers. However, 2D NMR spectra were essential to confirm that the sample analyzed was the (-)-CBD enantiomer. It is clear the importance of analysis and interpretation of 2D NMR spectra of synthetic and natural can-nabinoids, to guarantee the optical and three-dimensional purity of these substances and allow safe advances in science, medicine, and industry.