INTRODUCTION
Parasitic diseases are a heterogeneous group of infections which may be caused by protozoa, helminths or ectoparasites [1]. International travel and shifting patterns of immigration have increased the importance of awareness of the major clinical syndromes associated with infections due to parasites [2]. Protozoan parasitic infections transmitted by insect vector pose a significant global health problem [3], such Chagas diseases and Leishmaniasis.
Chagas disease, a vector-borne parasitosis caused by Trypanosoma cruzi [4], the great number of people infected with T. cruzi and the millions in risk of being infected together with the low efficacy of the actual treatments make of this disease one of the major health problems in Latin America [5]. Chagas disease has two successive phases: acute and chronic. Acute T. cruzi infection leads to focal myocarditis with accompanying necrosis of infected myocytes and reparative interstitial fibrosis. During the chronic disease, parasitaemia drastically decreases and parasites are barely detected [6]. Only two drugs, the 2-nitroimidazole benznidazole and the 5-nitrofuran nifurtimox, are licensed for the treatment of Chagas disease, although their safety and efficacy profile are far from ideal [7].
Leishmaniasis is a disease caused by intracellular protozoan parasites of the genus Leishmania [8]. The three main clinical manifestations of this illness are cutaneous, mucocutaneous and visceral [9]. The control of Leishmaniasis has been based on che motherapy with pentavalent antimonials for more than 70 years. Meglumine anti-moniate is a first-line drug, but use of this therapeutic is limited by its high cost and toxicity [10].
The impact of natural products on drug discovery is considerable, not only for cancer but also for parasitic infections [11]. Naphthoquinones constitute a structurally diverse class of phenolic compounds with wide applications in the fields of pharmacy and medicine [12] and are of key importance in organic synthesis and medicinal chemistry. In the last few years, various synthetic routes have been developed to prepare bioactive compounds derived [13]. The biological activity of quinones has been related to their redox properties and their capacity to accept one or two electrons to form the corresponding radical-anion (Q.-) and hydroquinone radical dianion (Q2-). These intermediate species interact with crucial cellular molecules such as oxygen, DNA and proteins modifying their biological activity [14]. Beyond these important features, the T. cruzi mitochondrion is known to be deficient in reactive oxygen and nitrogen species detoxification, being especially sensitive to oxidative stress conditions [15].
The biological activity of 1,4-naphthoquinones derivatives have been reported to exhibit a variety of pharmacological properties involving antimicrobial [16], anticancer [17], antioxidant [18], trypanocidal [19], leishmanicidal [20], anti-inflammatory [21], herbicidal [22], antiplasmodial [23], fungicidal [24].
Considering a variety of resistance mechanisms to antimonials in Leishmania [9], a limited efficacy of agents therapeutics in the treatment of Chagas disease, and investigation by different research groups to discover new molecules that can be used such strategies for Chagas disease and leishmaniasis chemotherapy. Our lab has been interested in the synthesis and pharmacological properties of 1,4-naphthoquinone derivatives. Thus, we synthesized nine compounds to evaluate their leishmanicidal, trypanocidal activity and cytotoxic properties.
MATERIALS AND METHODS
Chemistry
The carboxylic acids 2a-d and 7 were purchased from Sigma-Aldrich and used without further purification. The reagents 4 and 8 were obtained from cardanol as described in the literature [25, 35]. Purifications of compounds were made by column chromatography (CC) using Merck silica gel 60 (230 e 400 mesh) and a mixture of hexane and ethyl acetate was used for elution. All the reactions were monitored by TLC using Merck silica gel 60. 1H (300 MHz) and 13C NMR (75 MHz) spectra were recorded with a Bruker DPX-300 spectrometer using CDCl3, or DMSO-d6 as the solvent with TMS as an internal standard. Chemical shifts are reported in ppm and coupling constants ( J) in Hertz. Mass spectra (EI, 70 eV) were run on a Shimadzu CGMS QP2010 Plus gas chromatography mass spectrometer. The main fragments were described as a relation between atomic mass units and the charge (m/z) and the relative abundance in percentage of the base-peak intensity. Infrared spectra were recorded on Perkin-El-mer model 783 in a KBr cell for liquid (film) or KBr pellets for solids and the absorption wave numbers expressed in cm-1. HRMS was acquired using a UFLC Shimadzu LC-20AD apparatus, with and IES-Q-QTOF-microTOF III detector (Bruker Dalto-nics) in chemical ionization positive ion mode (m/z 120-1200). Melting points were determined by MQAPF-301 model equipment.
General procedure
The general procedure for the synthesis of 1,4-naphthquinone derivatives (3a-3d) was as described in literature [26, 32]. A solution of 1 g (NH4)2S2O8 in H2O (10 mL) was added dropwise over 90-120 min to a stirred suspension of H2O (100 mL), CH3CN (20 mL), AgNO3 (0.25 g), 2-Bromo-[1,4]naphthoquinone (0,2 g) and 1,5 mmol car-boxylic acid (Palmitic, linoleic, 3-(4-Methoxy-phenyl)-propionic, oleic) at 65-75 °C. The resulting mixture was stirred for another 30 min. Then the material was brought to room temperature, washed with NaHCO3 and dried with MgSO4, was filtered, concentrated under reduced pressure and purified by flash chromatography on silica gel with hexane/ethyl acetate.
2-Bromo-3-pentadecyl-[1,4]naphthoquinone (3a)
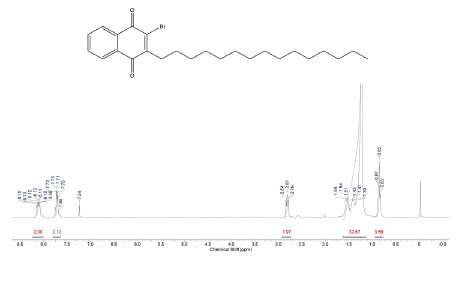
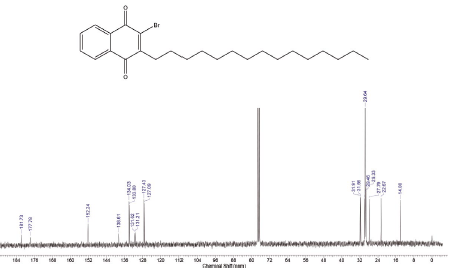
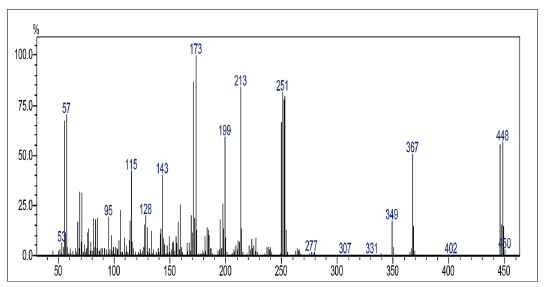
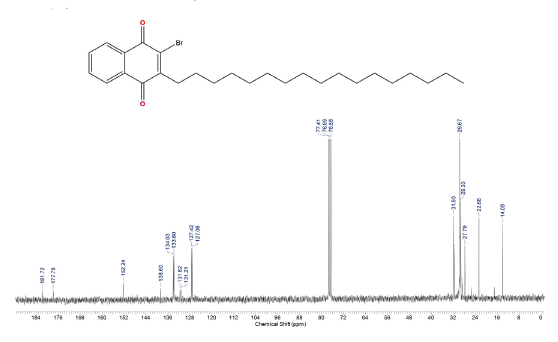
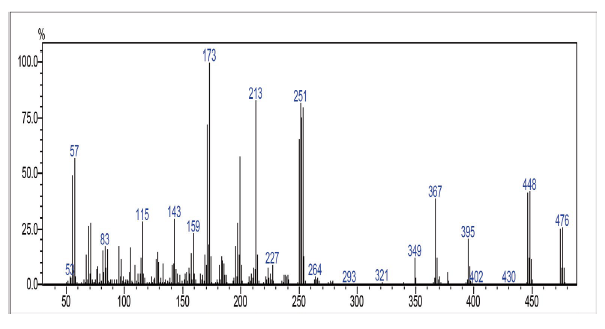
This compound was prepared according to general procedure. Yield: 66 % (210 mg); Yellow oil. 1H NMR (300 MHz, CDCl3): δ = 0.85 (t, J = 9.0 Hz, 3H, CH3), 1.00-1.50 (m, 24H, CH2), 1.54 (m, 2H, CH2), 2.81 (t, J = 9.0 Hz, 2H, CH2), 7.08-7.73 (m, 2H, CH), 8.06-8.15 (m, 2H, CH). 13C NMR (75 MHz, CDCl3): S = 14.0 (CH3), 22.6 (CH2), 27.7 (CH2), 31.6 (CH2), 29.0-30.0 (CH2), 31.9 (CH2), 127.0 (CH), 127.4 (ch), 131.2 (C), 131.6 (C), 133.8 (CH), 134.0 (CH), 138.6 (C), 152.2 (C), 177.7 (c=O), 181.7 (C=O). MS (EI+): m/z (%) 448,30 [M+●+2] (56,91); 447,30 [M+●+1] (55,97); 446,30 [M+●] (16,05); 253,05 (79,96); 252,00 (77,94); 251,05 (82,13); 173,10 (100,00). HRMS (ESI): m/z = not ionizable [M+H]+.
2-Bromo-3-heptadecyl-[1,4]naphthoquinone (3b)
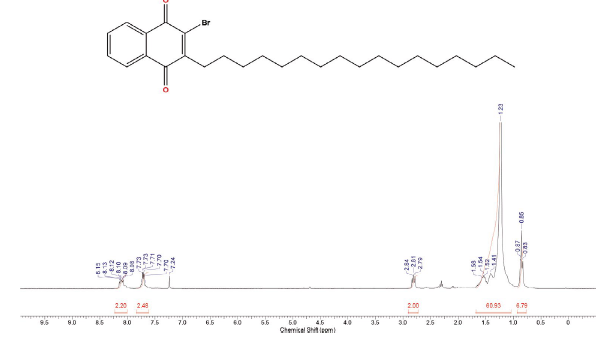
Compound was prepared according to general procedure. Yield: 47 % (187 mg), yellow solid, mp = 58-59 °C. 1H NMR (300 MHz, CDCl3) δ: 0.85 (t, J = 6.0 Hz, 3H, CH3); 1.00-1.70 (m, 30H, CH2); 2.81 (t, J = 9.0 Hz, 2H, CH2); 7.68-7.76 (m, 2H, CH); δ 7.68-7.76 (m, 2H, CH). 13C NMR (75 MHz, CDCl3) S: 14,0 (CH3); 22,6 (CH2); 27,7 (CH2); 28,0-30,0 (CH2); 31,6 (CH2); 31,9 (CH2); 127,0 (CH); 127,4 (CH); 131,2 (C); 131,6 (C); 133,8 (CH); 134,0 (CH); 138,6 (C); 152,2 (C); 177,7 (C=O); 181,7 (C=O). MS (EI+): m/z (%): 474 [M+●] (25); 476 [m+●+2] (26); 475 [M+●+1] (7); 448 (42); 447 (12); 446 (41); 253 (80); 252 (75); 251 (82); 173 (100). HRMS (eSI) m/z: not ionizable [M+H]+.
2-Bromo-3-[2-(4-methoxy-phenyl)-ethyl]-[1,4]naphthoquinone (3c)
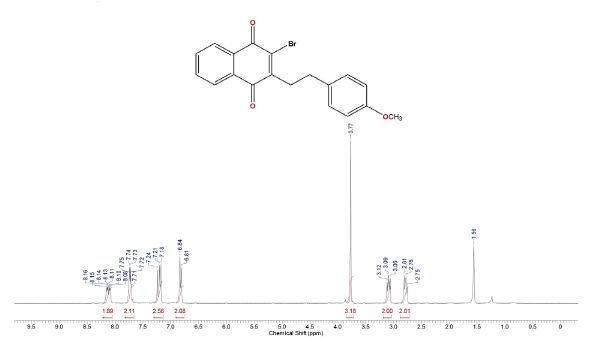
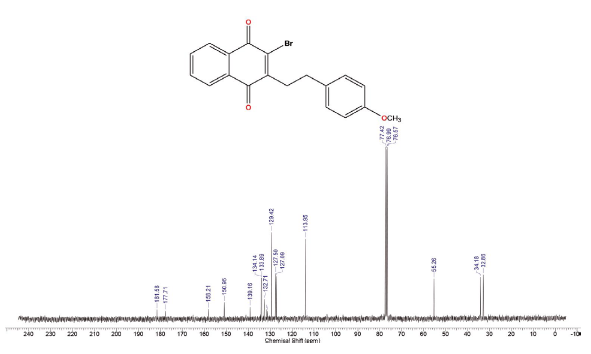
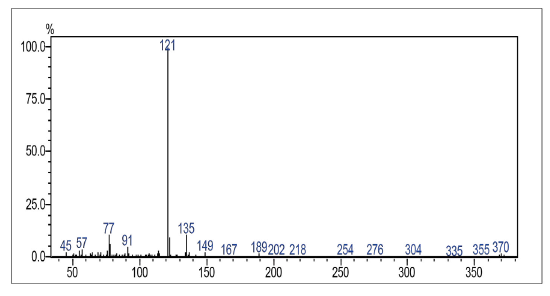
Compound was prepared according to general procedure. Yield: 10 % (20 mg), yellow oil. 1H NMR (300 MHz, CDCL,) δ: 2.78 (t,J = 9.0 Hz, 2H, CH2); 3.08 (t,J = 9.0 Hz, 2H, CH2); 3.77 (s, 3H, OCH3); 6.82 (d, J = 9.0 Hz, 2H, CH); 7.19 (d, J = 9.0 Hz, 2H, CH); 7.71-7.76 (m, 2H, CH); 8.08-8.16 (m, 2H CH). 13C NMR (75 MHz, CDCL3) δ: 32.8 (CH2), 34.1 (CH2), 56.2 (OCH3), 113.9 (CH), 127.0 (CH), 127.5 (CH), 129.4 (CH), 131.1 (C), 131.5 (C), 132.7 (C), 133.8 (CH), 134.1 (CH), 139.1 (c), 150.9 (C), 158.9 (C), 177.7 (C=O), 181.5 (C=O). MS (EI+): m/z (%): 370 [M+●] (2); 371 [M+●+1] (0,5); 372 [M+●+2] (1); 135 (19); 121 (100); 77 (13). HRMS (ESi) m/z: not ionizable [M+H]+.
2-Bromo-3-heptadecyl-8(Z)-enyl-[1,4]naphthoquinone (3d)
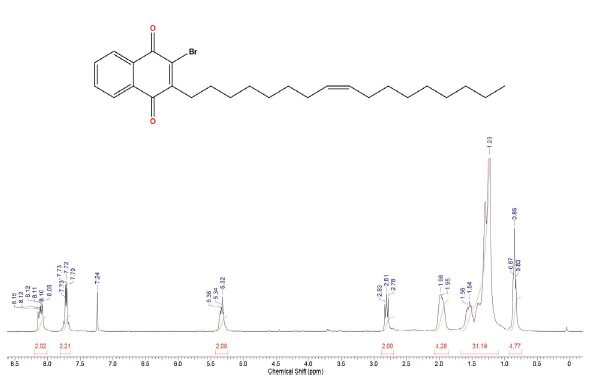
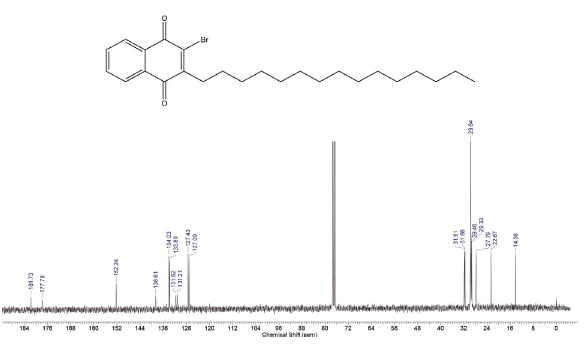
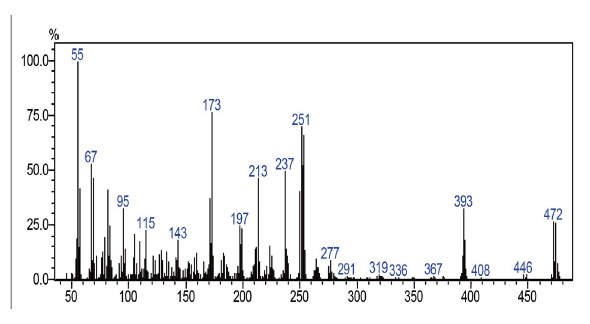
Compound was prepared according to general procedure. Yield: 10 % (40 mg), yellow oil. 1H NMR (300 MHz, CDCl3) δ: 0.85 (t,J = 9.0 Hz, 3H, CH3), 1.10-1.50 (m, 20H, CH2), 1.54 (qt, J = 9.0 Hz, 2H, CH2), 1.90-2.10 (m, 4H, CH2), 2.81 (t, J = 9.0 Hz, 2H, CH2), 5.27-5.40 (m, 2H, CH), 7.70-7.73 (m, 2H, CH), 8.08-8.15 (m, 2H, CH). 13C NMR (75 MHz, CDCL3) δ: 14,0 (CH3); 22,6 (CH2); 27,1 (CH2); 27,2 (CH2); 27,7 (CH2); 28,0-30,0 (CH2); 31,6 (CH2); 31,8 (CH2); 127,0 (ch); 127,4 (CH); 129,7 (CH); 130,0 (CH); 131,2 (C); 131,6 (C); 133,8 (CH); 134,0 (CH); 138,6 (c); 152,2 (C); 177,7 (C=O); 181,7 (C=O). MS (EI+): m/z (%): 472 [M+●] (22); 473 [M+●+1] (8); 474 [M+●+2] (21); 253 (58); 252 (45); 251 (61); 55 (100). HRMS (ESI) m/z: not ionizable [M+H]+.
N-(1,4-dioxo-1,4-dihydro-naphthalen-2-yl)-3,4,5-trimethoxy-benzamide (6a)
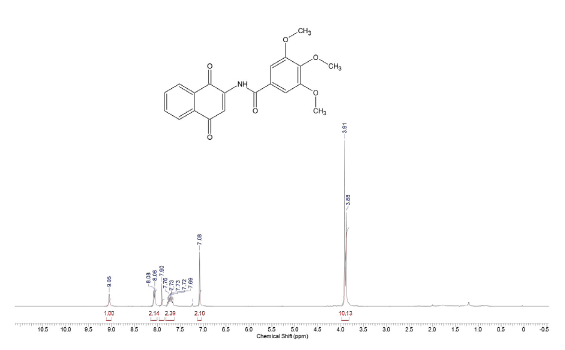
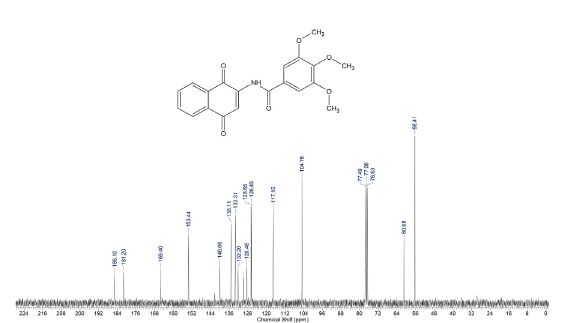
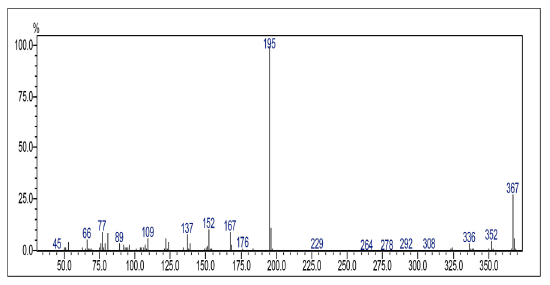
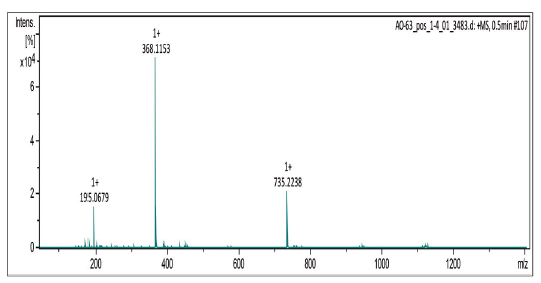
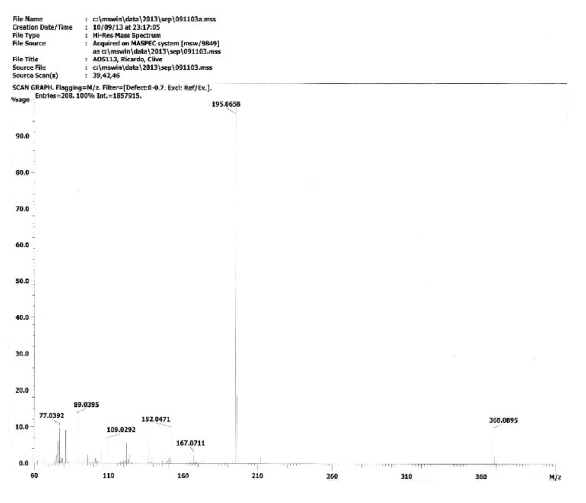
Compound was prepared as described previously in literature [27]. 2-amino-1,-4-naphthoquinone (6) (215 mg, 1.2 mmol) was dissolved in freshly distilled THF (15 mL). NaH (100 mg, 4.2 mmol) was added to the solution and the mixture was stirred at room temperature for 30 mins. 3,4,5-trimethoxy-benzoyl chloride (0.27 mL, 2.14 mmol) was added and the mixture was stirred for 5 mins. THF was removed under vacuum and the mixture was washed with ice-water (10 g ice and 10 mL water). The ice-water mixture was extracted with CH2Cl2 (30 mL, 20 mL consecutively). The combined organic phase was washed with water (3 x 20 mL), saturated NaCl solution (3 x 20 mL), and then dried over anhydrous MgSO4. The crude material was purified via column chromatography. Yield: 20 % (89 mg), yellow solid, mp = 167-168 °C. 1H NMR (300 MHz, CDCL3) δ: 3.91 (s, 6H, CH3), 3.88 (s, 3H, CH3), 7.08 (s, 1H, CH), 7.69-7.76 (m, 2H, Ch), 8.06-8.08 (m, 2H, CH), 9.05 (s, 1H, NH). 13C NMR (75 MHz, CDCL3) S: 56.4 (OCH3), 60.9 (OCH3), 104.7 (CH), 117.1 (CH), 126.4 (CH), 126.6 (CH), 128.4 (C), 129.9 (C), 132.2 (C), 133.3 (CH), 135.1 (CH), 140.0 (C), 142.2 (C), 153.4 (C), 165.4 (C), 181.2 (C=O), 185.1 (C=O). MS (EI+): m/z (%): 77,10 (9,24); 152,10 (10,81); 167,15 (9,69); 195,10 (100,00); 367,20 [M+●] (27,60). HRMS (ESI) m/z calcd for C20H17NO6 +H: 368,1134, Found: 368,1153 [C20H17NO6+H] +.
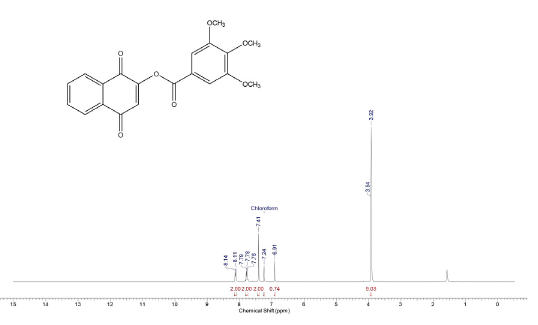
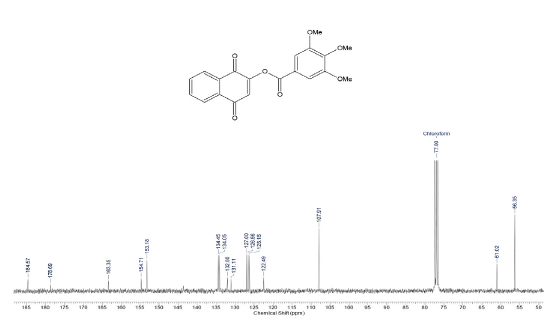
3,4,5-trimethoxy-benzoic acid 1,4-dioxo-1,4-dihydro-naphthalen-2-yl ester (9a)
Compound was prepared as described previously in literature [28]. To a solution of 200 mg (1,1 mmol) of 2-Hydroxy-1,4-naphthoquinone (9) in CH2Cl2 (50 mL), 40 mL of aqueous NaOH solution (10 %) were added and the solution was stirred for 4 h at room temperature. Then 330 mg (1,4 mmol) of the 3,4,5-trimethoxy-benzoyl chloride (8) were added and stirring was maintained for 48 h. The reaction mixture was washed with water (3 x 50 mL) and the organic phase was dried over MgSO4 and evaporated under reduced pressure. The crude material was purified via column chromatography. Yield: 94 % (380 mg), yellow solid, mp = 138-140 °C. 1H NMR (300 MHz, CDCL3) δ: 3.92 (s, 6H, OCH3), 3.94 (s, 3H, OCH3), 6.91 (s,1H, CH), 7.41 (s, 2H, CH), 7.76-7.79 (m, 2H, Ch), 8.11-8.14 (m, 2H, CH). 13C NMR (75 MHz, CDCL3) S: 56.3 (OCH3), 61.0 (OCH3), 107.9 (2CH), 122.4 (c), 126.1 (CH), 126.5 (CH), 127.0 (CH), 131.1 (C), 132.0 (C), 134.0 (CH), 134.4 (CH), 153.1 (3C), 154.7 (C), 163.3 (C), 178.6 (C), 184.5 (C). MS (EI+): m/z (%): 368 [M+●] (7); 196 (11); 195 (100); 167 (7). HRMS (EI) m/z calcd for C20H1607: 368,0896 Found: 368,0895 [C20H1607]+.
2-[7-(3-Methoxy-phenyl)-heptyl]-[1,4]naphthoquinones (5a)
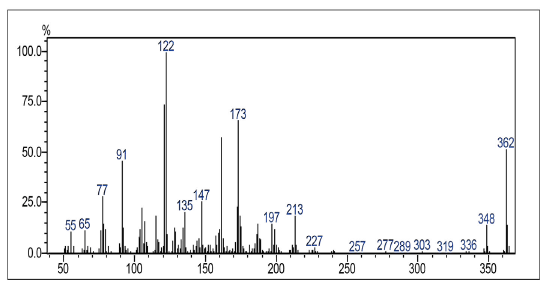
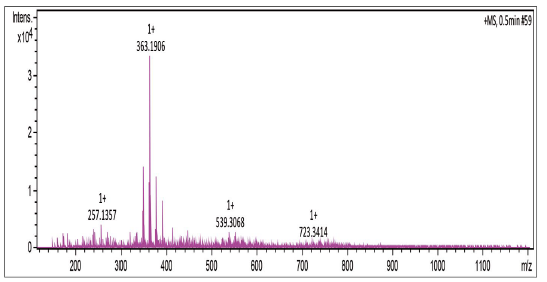
Compound was prepared according to general procedure with 1,4-naphthoquinone 5 and carboxylic acid 8. Yield: 40 % (188 mg), yellow oil. 1H NMR (300 MHz, CDCL3) δ: 1.10-1.70 (m, 10H, CH2), 2.55 (m, 4H, CH2), 3.77 (s, 3H, CH3), 6.69-6.73 (m, 3H, CH), 6.76 (s, 1H, CH), 7.16 (t, J = 6.0 Hz, 1H, CH), 7.67-7.72 (m, 2H, CH), 8.028.09 (m, 1H, CH). 13C NMR (75 MHz, CDCl3) S: 27.9 (CH2), 29.0-30.0 (CH2), 31.2 (CH2), 35.9 (CH2), 55.1 (CH3), 110.8 (CH), 114.1 (CH), 120.8 (CH), 126.0 (CH), 126.5 (CH), 129.1 (CH), 132.1 (C), 132.3 (C), 133.5 (CH), 133.5 (CH), 134.7 (CH), 144.4 (C), 151.9 (C), 159.5 (C), 185.2 (C). MS (EI+): m/z (%): 362 [M+●] (52); 173 (65); 122 (100); 91 (45). HRMS (ESl) m/z calcd for C24H2603 + H: 363,1916, Found: 363,1906 [C24H2603 + H]+.
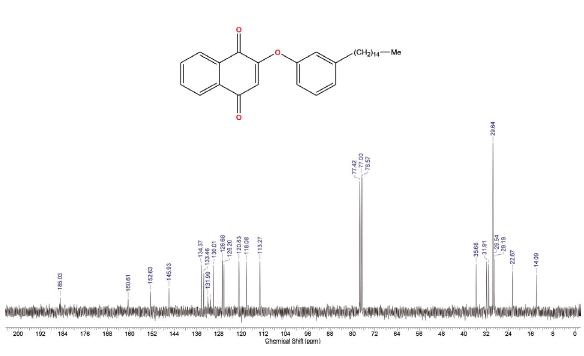
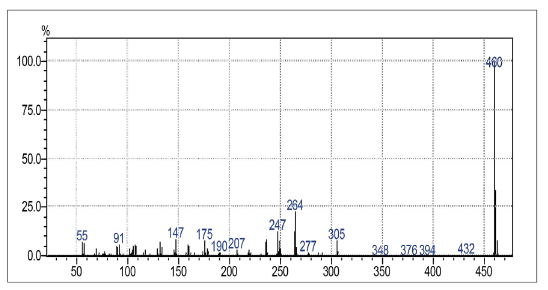
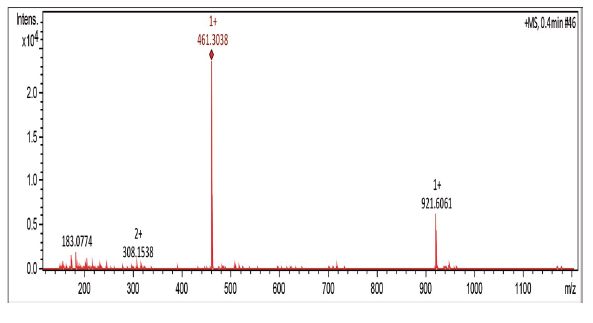
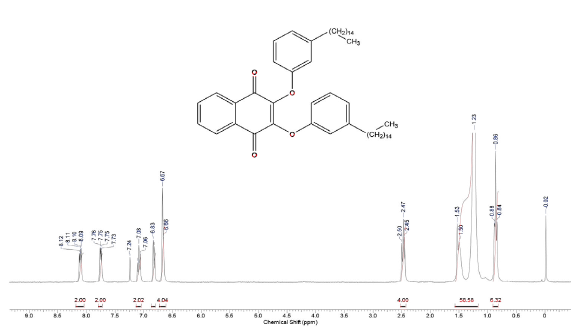
2-(3-Pentadecyl-phenoxy)-[1,4]naphthoquinone (4a) and 2,3-Bis-(3-pentadecyl-phenoxy)-[1,4]naphthoquinone (4b)
The Compounds were prepared as described previously in literature [29, 30]. A stirred mixture of 3-Pentadecyl-phenol 4, (128 mg, 0.4 mmol) and DMF (10 mL). After 1 min, K2CO3, (175 mg, 1.3 mmol) was added and the reaction mixture was stirred for 1 h at room temperature. 2-Bromo-1,4-naphthoquinone 1, (100 mg, 0.42 mmol) was added. After standing for 3 h, the mixture was treated with cold water. The reaction mixture was washed with water (3 x 50 mL) and extracted with hexane. The combined organic layers were dried over MgSO4, filtered and evaporated under reduced pressure. The residue was purified via preparative silica gel plates GF - 500 microns UniplatTM using hexane/ethyl acetate 8:1 to give 4a and 4b. The fraction with Rf = 2.6 afforded 20 mg (12 % yield) of a yellow solid, which was identified as 2-(3-Pentadecyl-phenoxy)-[1,4]naphthoquinones (4a), mp 50 - 51 °C. 1H NMR (300 MHz, CDCl3) δ: 0.85 (t, J = 6.0 Hz, 3H, CH3), 1.00-1.70 (m, 26H, CH2), 2.61 (t, J = 9.0 Hz, 2H, CH2), 5.95 (s,1H, CH), 6.91 (d, J = 9.0 Hz, 1H, CH), 6.93 (s, 1H, CH), 7.09 (d, J = 9.0 Hz, 1H, CH), 7.33 (t, J = 9.0 Hz, 1H, CH), 7.72-7.75 (m, 2H, CH), 8.03-8.20 (m, 2H, CH). 13C NMR (75 MHz, CDCl3) δ: 14.0 (CH3), 22.6 (CH2), 28.0-30.0 (CH2), 31.1 (CH2), 31.9 (CH2), 35.6 (CH), 113.2 (C), 118.0 (CH), 120.8 (CH), 126.2 (cH), 126.6 (CH), 130.0 (CH), 131.1 (C), 131.9 (CH), 133.4 (CH), 134.3 (CH), 145.9 (C), 152.6 (C), 160.6 (c), 185.0 (C). MS (EI+): m/z (%): 460 [M+●] (100); 305 (8); 264 (23); 247 (13). HRMS (ESI) m/z: calcd for C31H4003 +H: 461.3055, Found 461.3038 [C31H4003 + H]+. The fraction with Rf = 2,9 afforded 30 mg (10 % yield) of a yellow solid, which was identified as 2,3-Bis-(3-pentadecyl-phenoxy)-[1,4]naphthoquinone (4b), mp 58 - 59 °C. 1H NMR (300 MHz, CDCl3) S: 0.86 (t, J = 6.0 Hz, 6H, CH3); 1.00-1.60 (m, 52H, CH2); 2.47 (t, J = 6.0 Hz, 4H, CH2); 6.66 (d, J = 6.0 Hz, 2H, CH); 6.67 (s, 2H, CH); 6.82 (d,J = 6.0 Hz, 2H, CH); 7.08 (t, J = 6.0 Hz, 2H, CH), 7.73-7.76 (m, 2H, CH), 8.09-8.12 (m, 2H, CH). 13C NMR (75 MHz, CDCl3) δ: 14.1 (CH3), 22.6 (CH2), 29.0-30.0 (CH2), 31.1 (CH2), 31.9 (CH2), 35.7 (CH2), 113.6 (CH), 116.6 (CH), 123.6 (CH), 126.7 (cH), 128.9 (CH), 130.8 (C), 134.1 (CH), 144.7 (C), 146.0 (C), 156.4 (c), 180.5 (C). MS (EI+): m/z (%): 763 [M+●] (15); 762 (27); 460 (13); 108 (100); 57 (29). HRMS (ESI): m/z = not ionizable [M+H]+.
Biological activities
Parasites and Cell Cultures
The experiments of anti-parasitic activity were performed with the Y strain of Trypanosoma cruzi (epimastigotes) and promastigotes of Leishmania amazonensis (WHOM/BR/75/JOSEFA strain). Epimastigote forms were cultivated in Liver Infusion Tryp-tose (LIT) medium supplemented with 10 % heat-inactivated fetal bovine serum (FBS; Gibco Invitrogen, Grand Island, NY, USA), kept at 28 °C, and maintained by weekly transfers. The promastigote forms of L. amazonensis were maintained in culture at 25 °C with weekly transfers to fresh Warren's medium supplemented with 10 % FBS. In order to assess the cytotoxicity of the compounds, LLCMK2 cells (Macaca mulatta epithelial kidney cells) were maintained in Dulbecco's modified Eagle medium (DMEM; Gibco Invitrogen), pH 7.4, supplemented with 2 mM L-glutamine, 10 % FBS, and 50 mg/L gentamicin at 37 °C in a humidified 5 % CO2 atmosphere.
Anti-proliferative Activity against Epimastigote Forms (Trypanosoma cruzi)
Epimastigotes (1x106 parasites/mL) in the exponential phase of growth (96 h) were harvested and incubated in the presence of LIT supplemented with 10 % FBS added or not to increasing concentrations of the drug candidates. Parasites incubated at 28 °C in 96-well flat-bottom plates were afterwards counted in a Neubauer hemocyto-meter under light microscopy. The IC50 (concentration that inhibited 50 % of parasite growth) were determined by regression analysis of the data.
Anti-proliferative Activity against Promastigote Forms (Leishmania amazonensis)
Promastigotes (1 x 106 cells/mL) at exponential phase of growth (48 h cultures) were inoculated in a 96-well plate in the absence or presence of different concentrations of the drug candidates. Activity against promastigote forms was evaluated after 72 h by using the XTT method, which consists on the incubation of the cultures on the presence of a combination of the tetrazolium compound 2,3-Bis-(2-Methoxy-4-Ni-tro-5- Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide (XTT, Sigma) and the electron coupling reagent phenazine methosulphate (PMS, Sigma). After the treatment of the parasites, 100 µL of the mixture of XTT (0,5 mg/mL) and PMS (0,06 mg/mL) was added to each well, the plate was incubated for 4 h protected from light at 28 °C and the absorbance measured at 450 nm in a microplate reader (Bio Tek - Power Wave XS). By comparing the absorbance in control untreated parasites with the treated ones, the inhibitory activity was determined. The IC50 (concentration that inhibited 50 % of parasite growth) were determined by regression analysis of the data.
Cytotoxicity Assay
To evaluate the cytotoxicity of the compounds, the MTT assay was applied as previously described [31]. This colorimetric assay is based on the ability of viable mitochondria to convert MTT, a water-soluble tetrazolium salt (3-[4,5-dimethylthiazol-2-yl]-2,5-di-phenyltetrazolium bromide), into an insoluble purple- colored formazan precipitate. LLCMK2 cells were collected from confluent cultures, plated in 96-well plates, and incubated at 37°C in a humid 5 % CO2 atmosphere. After 24 h, the medium was replaced with new DMEM that contained concentrations of the compounds that ranged from 3.7 to 73.6 µM. Following 96 h incubation, the cells were washed in PBS, and 50 µL of MTT (2 mg/mL) was added to each well. The formazan crystals were solubilized in DMSO, and absorbance was read at 570 nm in a microplate reader (Bio Tek - Power Wave XS). The concentration that diminished 50 % of the absorbance value observed in the control represented the CC50 (cytotoxic concentration for 50 % of the cells).
Statistical Analysis
All of the quantitative experiments were conducted in at least three independent experiments in duplicate. The statistical analyses were performed using GraphPad Prism 5.0 software. The data were analyzed using one-way analysis of variance (Anova), and the Tukey post hoc test was used to compare means when appropriate. Values of p, 0.05 were considered statistically significant.
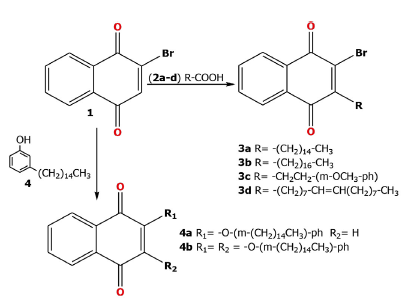
The 1,4-naphthoquinones derivatives 3a-d (Scheme 1) were obtained from compound 1 by coupling reaction with carboxylic acid 2a-d (Palmitic, linoleic, 3-(4-Methoxy--phenyl)-propionic, oleic) using ammonium persulfate ((NH4)2S2O8) and silver nitrate (AgNO3), following conditions previously reported [26; 32].
RESULTS AND DISCUSSION
Chemistry
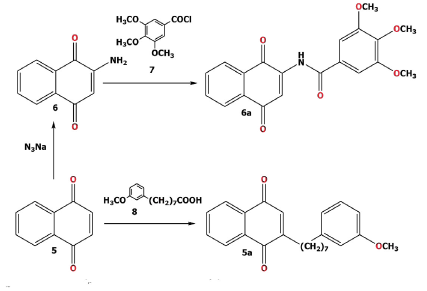
On the other hand, treatment of compound 1 with phenol 4 (saturated cardanol) obtained two compounds (4a-b; Scheme 1) by nucleophilic substitution of the bromo atom attached to the C-2 and for addition of phenol in C-3 using K2CO3 and DMF [29]. The compound 6a (Scheme 2) were obtained by reaction of compound 6 and 7 in NaH/THF as described in literature [27].
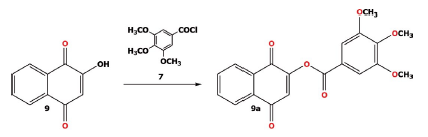
The 1,4-naphthoquinone (5) was used to prepare 5a as described previously [26; 32] with 8-(3-methoxyphenyl) octanoic acid 8. The condensation reaction of naphthoquinone 9 with 3,4,5-trimethoxybenzoyl chloride (7) in the presence of K2CO3 (Scheme 3), gave the compound 9a [28].
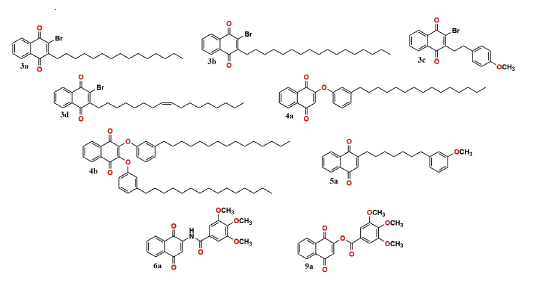
The 1,4-naphthoquinone derivatives (3a-d, 4a-b, 5a, 6a and 9a) were obtained in 10-94 % yields and were further fully characterized by 1H, 13C, mass spectrometry and HRMS, and the data are compatible with their structures, the analytical data for all compounds are shown in appendix.
In vitro biological activity
In the present study, nine 1,4-naphthoquinone derivatives (Figure. 1) were synthesized and investigated for their potential as trypanocidal and leishmanicidal agents.
In order to obtain preliminar information, the in vitro activity of compounds 3a-d, 4a-b, 5a, 6a and 9a was evaluted against epimastigote forms of T. cruzi and promastigote forms Y of L. amazonensis.
Trypanocidal activity of 1,4-naphthoquinones derivatives on epimastigotes proliferation
The synthesized 1,4-naphthoquinone derivatives 3a-d, 4a-b, 5a, 6a and 9a were evaluated against epimastigotes forms of T. cruzi.Table 1 shows IC50 values for the in vitro trypanocidal assay, the CC50 for the cytotoxicity to LLCMK2 cells (MTT method) and the SI for each compound.
Some of these compounds showed good biological activity against epimastigotes form of T. cruzi. Among them, compound 6a was the most potent of 1,4-naphthoquinone derivatives, showing an IC50 value of 0.25 ± 0.02 µM, being 35.0 times more active than Benznidazol (IC50 = 8.80 ± 0.40 µM) when assayed. However, the cytotoxicity of CC50 6.53 ± 2.69 (si = 26.12) was observed.
In 2013, Salomão et al. evaluated the efficacy of sixteen 1,4-naphthoquinones against the infective bloodstream trypomastigote forms of T. cruzi and the prototype 1,4-naph-thoquinone 5, demonstrated higher activity than Benznidazol (IC50 = 26.0 ± 4.0 [M) [33]. The compound 5 obtained an efficacy with IC50 = 0.79 ± 0.02 µM and was compared with other derivatives such 2-Bromo-1,4-naphthoquinone 1 (IC50 = 1.37 ± 0.03 µM) and 2-Hydroxi-1,4-naphthoquinone 9 (IC50 = 563.18 ± 83.28 µM). Among the most active compounds on trypomastigotes, the compound 5 was selected for further studies and was the most active against epimastigotes forms of T. cruzi with IC50 = 0.26 ± 0.05 µM. Comparing the compound 5 (IC50 = 0.26 ± 0.05 µM) and derivative 6a (IC50 = 0.25 ± 0.02 [M), we noted that the activity against proliferation of T. cruzi epimastigotes was equivalent, evidencing that amination reaction at the 2' position of the 1,4-napthoquinone followed of the N-acylation reaction with 3,4,5-tri-methoxybenzoyl chlorid had no pronounced trypanocidal action.
In addition, compound 9 (lawsone) as prototype was active on culture growth of the Tulahuén strain epimastigotes form of T. cruzi with IC50 = 20.0 ± 6.0 µM [34]. When we acylation reaction of lawsone with 3,4,5-trimethoxybenzoyl chlorid, obtaining the compound 9a, was observed the increased of the trypanocidal activity (IC50 = 5.06 ± 0.44 µM) in 4.0 times. Comparison between compounds 6a (IC50 = 0.25 ± 0.02 µM) and 9a (IC50 = 5.06 ± 0.44 µM) the activity is significantly increased, the presence of NH group had a positive effect on the trypanocidal activity.
Considering the structural of compounds 3a-d and when compared, the substitution of the alkyl or allyl chain (e.g. 3a, 3b, 3d) for phenyl ring with methoxy groups (e.g. 3c) result in increased trypanocidal activity. It is interesting to note that increase in the length of the carbon chain decrease activity, the compound 3b (IC50 = 9.46 ± 4.75 µM) was 2.0 times less active than the compound 3a (IC50 = 4.58 ± 0.47 µM). In contrast, the comparison of the compounds 3b (IC50 = 9.46 ± 4.75 µM) and 3d (IC50 = 4.01 ± 1.19 µM), the presence of a double bond in the side chain increase the activity. Comparing the activity of compound precursor 1 (IC50 = 1.37 ± 0.03 µM) with 3c (IC50 = 0.71 ± 0.32 µM), the effect of methoxy group resulted in an increase in activity.
The activities of the compounds 4a (IC50 = 87.59 ± 20.72 µM) and 4b (IC50 > 100 µM) gave low activity.
Table 1 Values of activities for 1,4-naphthoquinone derivatives 3a-d, 4a-b, 5a, 6a and 9a, in vitro Trypanocidal activity (IC50 µM) against epimastigote form T. cruzi, cytotoxicity (CC50 µM, LL-CMK2 cells) and selectivity index (SI).
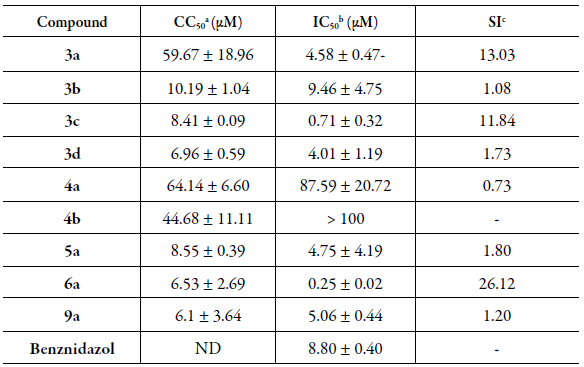
aCC50: concentration that kills 50 % of LLCMK2 cells, 96 h after incubation with the compounds determined by the MTT method. bIC50: concentration that inhibits 50 % of the parasite growth in relation to control cultures with no drugs. cSI: Selectivity Index ¼ CC50/IC50. ND: not determined.
Leishmanicidal activity
All the synthesized compounds were tested against promastigote form L. amazonensis (Table 2) and only compounds 3c, 3d, 5a, 6a and 9a exhibited activity leishmanicidal with values between 0.16 and 2.49 µM. In terms of the most active of the naphthoquinones derivatives, compound 3c showed the best IC50 0.16 ± 0.05 µM, but is less active in comparison with the reference compound (Amphotericin B, IC50 = 0.06 µM).
Table 2 Values of activities for 1,4-naphthoquinone derivatives 3a-d, 4a-b, 5a, 6a and 9a, in vitro Leishmanicidal activity (IC50 µM) against promastigote form L. amazonensis, cytotoxicity (CC50
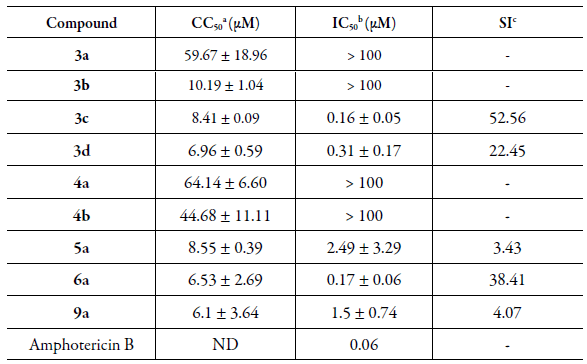
aCC50: concentration that kills 50 % of LLCMK2 cells, 96 h after incubation with the compounds determined by the MTT method. bIC50: concentration that inhibits 50 % of the parasite growth in relation to control cultures with no drugs. cSI: Selectivity Index ¼ CC50/IC50. ND: not determined.
CONCLUSIONS
In conclusion, the synthesis of 1,4-napthoquinone derivatives was prepared and biologically assayed against T. cruzi and L. amazonensis in order to obtain an action profile for these compounds. The observed results were considered as preliminary and the active compounds 3a, 3c, 3d, 5a, 6a and 9a showed moderate to excellent trypanocidal activity. In particular, the compound 6a exhibited better activity against epimastigotes forms of T cruzi than benznidazole. On the other hand, the activity of 1,4-napthoquinone derivatives against promastigote forms L. amazonensis was not more effective than the Ampho-tericin B. Based on the result of this work, the compound 6a merits further studies in amastigotes and trypomastigotes to investigate the mechanism of trypanocidal action in both parasite forms and its potential for agents against Chagas disease.