INTRODUCTION
Diabetes is a metabolic disorder of multiple etiologies characterized by hyperglycemia resulting from defects in insulin secretion, insulin action or in both [1] which can induce non-enzymatic glycosylation of proteins, lipids, and nucleic acids, that lead to the formation of advanced glycation end products (AGEs) and development of chronic clinical complications, such as atherosclerosis, nephropathy, neuropathy and cataracts [2, 3].
Natural products represent an important option for the discovery and development of promising lead candidates with biological activity [4], because of the complexity of their secondary metabolism that results in a large chemical diversity [5, 6]. A promising approach in natural products research is bioassay-guided isolation and identification of lead compounds [7]. As a recent example, performed a bio-guided isolation from the ethanolic extract of Cordia morelosana in which allowed to obtain rosmarinic acid and methyl rosmarinate, responsible for activities observed in the extract [8].
Byrsonima is one of the major general of the family Malpighiaceae, including about 150 species with different biological properties reported, such as spasmogenic, immunostimulatory, anti-inflammatory, anti-hemorrhagic, anti-hyperlipidemic, antiulcer and antidiarrheal [9-11].
Byrsonima garcibarrigae Cuatrec., a member of this genus, is an endemic arboreal species in northern Brazil, one of the thousands of species in the Amazon region with pharmacological potential still under investigation. In a project of this research group, five species of Byrsonima genus were preliminarily screened against α-glucosidase, and B. garcibarrigae showed promising results. Thus, aiming to identify bioactive compounds, this study performed a phytochemical study bioassay-guided by a-glucosidase inhibition, additionally evaluating the antioxidant, anti-glycation activities of extracts and fractions from the stem barks of B. garcibarrigae.
METHODOLOGY
Plant material
The collection of plant samples was authorized by the Chico Mendes Institute for the Conservation of Biodiversity (ICMBio) of the Brazilian Ministry of the Environment, under the registration 41553-1. Plant material was collected at the Adolpho Ducke Manaus Forestry Reserve (Amazonas) and taxonomically identified, where a voucher specimen was deposited under number 691 by the Herbarium of the National Institute of Amazonia Research (INPA).
Preparation of the plant extract
Stem bark samples of B. garcibarrigae were dried at room temperature for 4 days followed by a period of 24 hours at 40 °C in an air circulating stove, then pulverized with a mechanical grinder. The extracts were obtained by maceration using 822 g of stem barks and portions of 700 mL of hexane (total of 6 L), ethyl acetate (total of 4 L) and methanol (total of 4 L), in cycles of 15 min, in an ultrasound bath (Ultrasonic Cleaner Unique®), sequentially named EHBG (2.88 g, 0.35%), EABG (5.67 g, 0.69%), and EMBG (111.46 g, 13.56%).
Total phenolic determination
The test was performed using the Folin-Ciocalteu colorimetric method [12]. Extracts (EHBG, EABG, and EMBG) were mixed with 50 μL of Folin-Ciocalteu reagent for 8 minutes in room temperature and 240 μL of sodium carbonate 0.4% was added. After incubation for 3 minutes at room temperature, the absorbance of the reaction mixture was measured at 750 nm. Gallic acid was used as a standard. The experiments were performed in triplicate and the mean results determined.
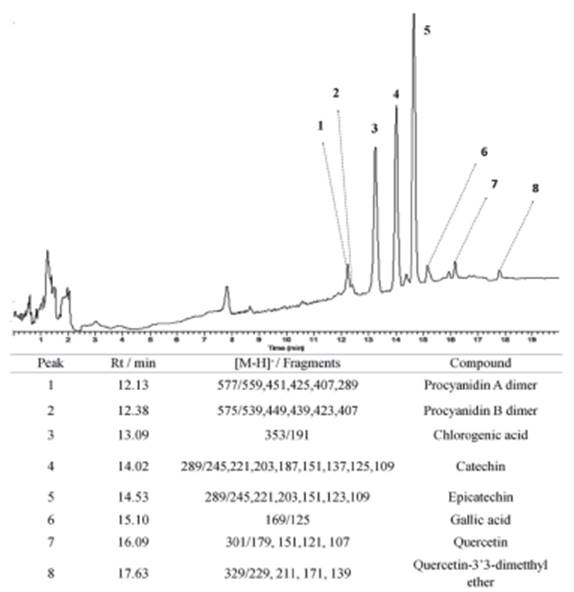
Instrumental Analysis
A HPLC-MS system consisting of an Accela 600 liquid chromatography system coupled to a TSQ Quantum Access triple-quadrupole (QqQ) mass spectrometer was employed for the chemical analyses (Thermo Fisher Scientific, Waltham, MA, USA). Electrospray ionization in negative mode (ESI) was applied to access the phenolic composition of B. garcibarrigae. Mass spectra (MS) were acquired at the m/z range from 50 to 1000, and tandem mass spectra (MS/MS) performed by collisional-induced dissociation of previously isolated precursor ions in the QqQ system. Argon was used as collisional gas. Tentative identifications were performed by manual interpretation of MS/MS spectral data with the literature.
Chromatographic separations were performed on a Kinetex C18 column (2.6 μm, 30 x 4.6 mm, 100 A pore size) (Phenomenex, Torrance, CA, USA) column using a binary mobile phase. Solvent A was ultrapure water, and solvent B was methanol. A gradient elution at 28 °C was performed as follows: 0-3 min, 58% B; 3-7 min, 58-100% at a flow rate of 0.4 mL/min. The autosampler temperature was held at 15 °C, and the injection volume was 2 μL. Ionization parameters were capillary voltage 4.5 kV, cone voltage 5 V, sheath gas 25 arb, and auxiliary gas 5 arb. Collision energies were applied as an increasing ramp from 2 to 50 eV.
1H NMR experiments were recorded in methanol-d4 (Cambridge Isotope Laboratories, Tewksbury, MA, USA) an INOVA 500 NMR spectrometer (Varian) operating at 500 MHz for 1H and at 125 MHz for 13C experiments, respectively. TMS (Cambridge Isotope Laboratories) was used as internal standard.
DPPH radical scavenging assay
The DPPH radical scavenging activity was performed according to Molyneux (2004) [13], with modifications. The assay was performed on a 96-well plate using 30 μL of extracts (EHBG, EABG, and EMBG) at 100 μg/mL and mixed with 270 μL of DPPH in ethanol (absorbance equal to 1 at 517 nm). The plate was kept in the dark for 30 minutes, after that the absorbance was measured at 517 nm. Blanks (DMSO) and standard (quercetin) were run simultaneously.
α-Glucosidase inhibition assay
This experiment used α-glucosidase from Saccharomyces cerevisiae (EC 3.2.1.20). Briefly, after the dilution of the extracts and fractions, the reaction was performed on microplate with the prepared enzyme solution (10 U/mL). Then, were obtained the values of the absorbance at 405 nm. With the addition of color reagent, readings were carried out every 5 min until the time of 30 min [14].
Fractionation from the methanolic extract
Fractionation of the EMBG was performed by liquid-liquid partition. For this purpose, 50 g of the extract was solubilized in 500 mL of methanol/water 1:1 and sequentially extracted with 300 mL of hexane and chloroform, and performed in triplicate, respectively. The obtained fractions were dried in a rotary evaporator (IKA® RV10 rotaevaporator basic), providing the following fractions: FHX and FCL. The solvent residual fraction, hydromethanolic (FHM), was eliminated using a mini spray dryer (MSD 1.0, Labmaq, Brazil).
The most active fraction, FHM (10 g), was fractioned over a polyamide-6-DF (Riedelde Haen, Seelze, Germany) column chromatography with an isocratic elution of methanol (column of 40 cm high and 6 cm diameter, and 288.4 g of as stationary phase). Fractions were collected into 100 mL flasks and evaluated by thin-layer chromatography (TLC) using silica gel 60 (ALUGRAM® Xtra SIL G, Macherey-Nagel) and ethyl acetate/toluene/formic acid/water 4:0.5:0.5:0.5 as mobile phase, which were grouped according to their similarities, affording 32 fractions.
All fractions were evaluated against α-glucosidase in enzymatic assays. F3 showed the highest inhibitory activity, therefore, it was submitted to preparative chromatography on glass plates with silica gel 60 (70-230 mesh) using chloroform/methanol (9:1) as a mobile phase. The compound was removed from the plates with silica and extracted with ethyl acetate yielding compound 1 (12 mg) and 2 (8 mg).
Epicatechin (1): 1H NMR (methanol-d4, 500 MHz) δH 2.5; 2.8 (dd, H-4); 4.0 (m, H-3); 4.6 (d, H-2); 4.8 (s, H-6); 5.6 (d, H-5'); 5.8 (d, H-2'); 6.75 (m, H-6'). ESI-MS (product ions, 15 eV) m/z: 289 [M-H]-, 245, 221, 203, 151, 123, 109.
Quercitrin (2): 1H NMR (methanol-d4, 500 MHz) δH 1,16 (d, J = 1,2 Hz, H-6''); 3,30 (m, H-4''); 3,54 (m, H-2''); 3,63 (m, H-3''); 4,08 (d, J = 1,2 Hz, H-1''); 4,22 (m, H-5''); 6,20 (d, J = 1,8 Hz, H-6); 6,42 (d, J = 1,8 Hz, H-8); 6,80 (d, J = 8,2 Hz, H-5'); 7,55 (dd, J = 2,1 e 8,5 Hz, H-6'); 7,68 (d, J = 2,1 Hz, H-2'). ESI-MS (productions, 15 eV) m/z: 447 [M-H]-, 301 [M-H Rha]-, 179, 151, 121.
Antiglycation activity
A mixture of bovine serum albumin (135 μL), glyoxal, fructose or glucose (135 μL) and different concentrations of EMBG, FHM, quercitrin and epicatechin (30 μL) was incubated at 37 °C and kept in the dark, the fluorescence of the samples was measured using an excitation of 340 nm and an emission of 420 nm. Aminoguanidine was used as positive controls and DMSO as negative control [15].
Docking molecular
Docking studies were performed employing the crystalline structures cocrystallized with known inhibitors available on the Protein Data Bank (PDB): α-glucosidase (PDB: 3W37) and receptor for advanced glycation endproducts (RAGE) (PDB: 3O3U). Water molecules and other molecules used in the crystallization of proteins not required for catalytic activity were removed in Pymol software (Schrödinger, Cambridge, USA), and all ligands were prepared and geometry, as well as energy, was minimized through ChemBioDraw program, and were subjected to energy minimization by the Merck Molecular Force Field (MMFF94).
The procedure of docking of ligands with the receptors has been performed using AutoDock Vina 1.1.2 (The Scripps Research Insitute, Jupiter, USA) [16]. Dimensions of grid box was 12x18x12 A and 14x10x10 A with grid spacing of 1.0 A for α-glucosidase and RAGE, respectively.
The results were visualized in the AutoDock Tools and the interactions were evaluated in terms of minimum binding energy (kcal/mol), number of hydrogen bonds, and other interactions formed between the active site residues of the macromolecule and ligand. 2D and 3D images of docking molecular results were obtained using Discovery Studio Visualizer 4.0 software (San Diego, CA, USA).
Statistical analysis
The results are presented as mean ± standard deviation, where applicable. All the results were compared by the Tukey test, considering values of p < 0.05 statistically significant. The median inhibitory concentrations (IC50) were calculated by the linear regression method. All the analyses were performed in GraphPad Prism 6 (San Diego, CA, USA).
RESULTS AND DISCUSSION
Since α-glucosidase inhibitors delay the release of glucose reducing postprandial hyperglycemia, they are an important approach to the treatment of type 2 diabetes [17]. Thus, this work studied the effect of B. garcibarrigae extracts on α-glucosidase, as well as their antioxidant potential, which is responsible for the development of long-term diabetic complication, and associates it with the increased risk of mortality and morbidity [18]. The inhibitory effects of the extracts against α-glucosidase were evaluated in comparison to those of acarbose, an antidiabetic drug (table 1).
Table 1 Total phenolic content, antioxidant capacity and inhibitory activity of crude bark extracts from B. garcibarrigae.
| Sample | Total phenolic content (%) | DPPH | α-glucosidase inhibition |
|---|---|---|---|
| IC50 value (μg/mL) | |||
| EHBG | 20.65 ± 1.43 | 130.42 ± 0.23 | 3.70 ± 0.61 |
| EABG | 28.41 ± 6.50 | 10.3 ± 0.03 | 3.21±2.05 |
| EMBG | 61.43 ± 0.50 | 9.2 ± 0.23 | 1.09 ± 0.32 |
| Quercetin | - | 3.4 ± 0.12 | - |
| Acarbose | - | - | 0.86 ± 0.10 |
All extracts inhibited α-glucosidase, being that inhibitory activity increased with increasing polarity even as the antioxidant activity. Thus, the activity of the extracts was solvent dependent. Extracts prepared by methanol had higher antioxidant properties, probably due to compounds possessing protonatable functional groups such as -COOH and -OH, such as some polyphenols, which are known antioxidants [19, 20].
Among all extracts, EMBG presented a greater inhibitory capacity and was fractionated. The FHX and FCL fractions were discontinued, because they were inactive against α-glucosidase (9.08±0.16%, and 11.0±0.79% of inhibition, respectively). On the other hand, FHM presented an IC50 of 1.02±0.49 μg/mL and was fractionated. Thirty-two fractions were obtained and were evaluated for their inhibitory activity, but only F3 and F4 showed promising inhibitory activity, which IC50 values of 91.45±0.94 and 62.04±0.65 μg/mL, respectively. The high activity of EMBG and FHM compared to fractions may be related to the that interactions by additive/non-interactive or synergistic effect of the compounds [21, 22]. In order to identify the compounds, the fraction F4 was subjected to preparative thin-layer chromatography and two compounds were isolated.
The NMR and MS fragmentation are in accordance with previously published data [23, 24] allowed the identification of epicatechin and quercitrin. These compounds had already been identified in other species of the genus Byrsonima, such as B. duckeana [25] and B. sericea [26], but are being reported for the first time in B. garcibarrigae.
Thus, due to its expressive antioxidant activity and for exhibit a potential inhibition of α-glucosidase, EMBG, FHM, and the isolated compounds were evaluated for inhibition of AGEs formation. In the body, increased levels of glucose and oxidative stress induce a non-enzymatic reaction between reducing sugars and proteins, lipids or nucleic acids, which is called glycation, which gives rise to AGEs [27].
Over time, mitochondrial respiratory chain proteins become increasingly glycated and mitochondrial DNA damage occurs, leading to a self-perpetuating cycle of AGE formation and oxidative stress independently of hyperglycemia [28]. For this, AGEs are associated with oxidative stress and inflammation, which are processes involved in the pathogenesis of many chronic diseases, such as diseases [29].
In figure 1 it is possible to observe that in the formation of AGEs via fructose, FHM and EMBG, epicatechin and quercitrin showed better inhibitions when compared to the aminoguanidine, a synthetic inhibitor of glycation. Aminoguanidine, epicatechin, quercitrin, FHM, and EMBG presented IC50 values of 29.26±0.13, 43.92±0.13, 7.42±0.23, 2.69±0.14 and 3.68±0.74 μg/mL, respectively.
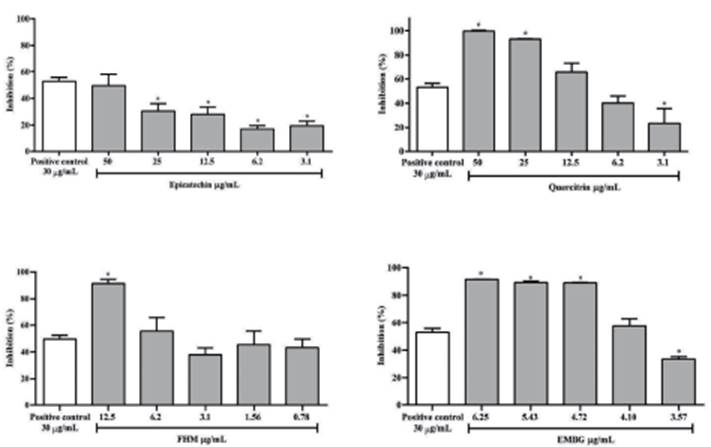
Figure 1 In vitro anti-glycation activity of extracts and compounds of B. garcibarrigae. The results are presented as mean ± standard deviation. p value versus Aminoguanidine 30 μg/mL (positive control): *p < 0.05.
It is worth mentioning that our work is the first to demonstrate the role of the extracts of B. garcibarrigae in the control of non-enzymatic glycation. Our findings suggest that the antioxidant, anti-α-glucosidase and anti-glycation capacities are related of the synergism between quercitrin, epicatechin and other compounds presents, since FHM was the more potent sample and showed some degree of affinity for the analyzed enzymes in silico. Quercitrin was the compound with the highest affinity (lower binding energy) for α-glucosidase which showed two hydrogen bonds with 3 residual amino acids Asp232, Asp357 and Arg552, while epicatechin was the compound with the highest affinity for RAGE which showed hydrogen bonds with 3 residual amino acids Glu44, Trp62 and Asp65 (figure 2). Both compounds showed affinity better than that of the co-crystallized inhibitor and showed affinity with both enzymes (table 2). In addition to the isolated flavonoids, 8 other phenolic compounds were identified in the EMBG (figure 3).
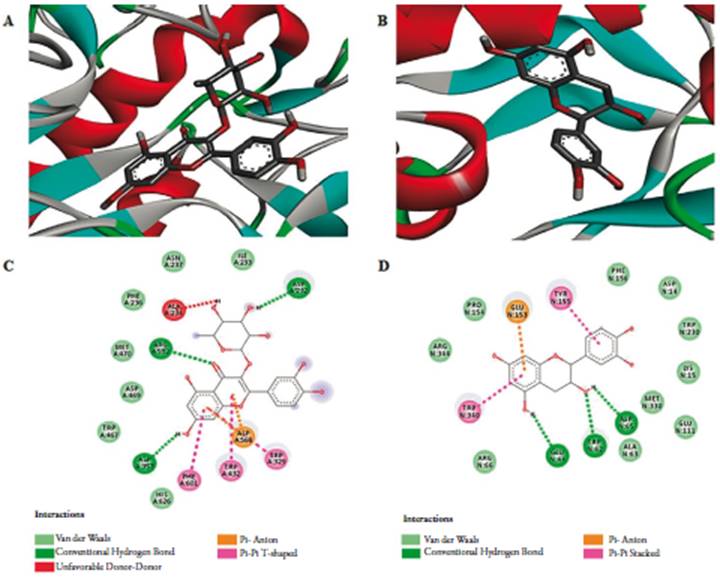
Figure 2 2D and 3D docking pose of ligands and their amino acid interactions in the active site. Crystal structure of α-glucosidase (PDB: 3W37) in complex with quercitrin (A and C) and RAGE (PDB: 3O3U) in complex with epicatechin (B and D).
Table 2 Energy of interaction (kcal/mol) between phenolic compounds and the enzymes RAGE and α-glucosidase.
| Compounds | α-glucosidase (PDB: 3W37) | RAGE (PDB: 303U) |
|---|---|---|
| Epicatechin | -7.4 | -8.7 |
| Quercitrin | -8.2 | -9.5 |
| Acarbose | -8.1 | - |
| Maltotriose | - | -9.4 |
Polyphenols have been reported to inhibit AGEs formation, such as chlorogenic acid, which acts by metal chelation and is an insulin-sensitizer, reducing the intestinal glucose absorption and its plasma peak [30]. Furthermore, decreases triglyceride contents in adipose tissue [31].
Other polyphenols such as procyanidins, which are the oligomeric or polymeric forms of epicatechin and catechin, significantly ameliorate postprandial hyperglycemia promoting GLUT4 translocation to the plasma membrane by activating both insulin- and AMPK-signaling pathways [32]. Besides that, epicatechin, catechin and the gallic acid act as α-glucosidase inhibitors [33, 34]. Quercetin and its derivatives exerts a significant hypoglycemic activity might by enhancing insulin sensitivity, promoting glycogen synthesis, inhibiting α-glucosidase activity and decrease the seriousness of numbness, jolting pain, and irritation for patients with type 2 diabetes neuropathy [35, 36].
Such findings base the inhibitory activity observed since high levels of phenolic compounds can inhibit enzymes by either interacting or inhibiting specific positions, as well as acting radical free radical scavenging [37, 38]. These evidences support our results and suggest of synergistic or additive effect between phenolic compounds identified (Figure 4) which can would interact with all enzymes analyzed and support a multi-target mechanism determined by the fraction components when compared isolated compounds [39, 40].
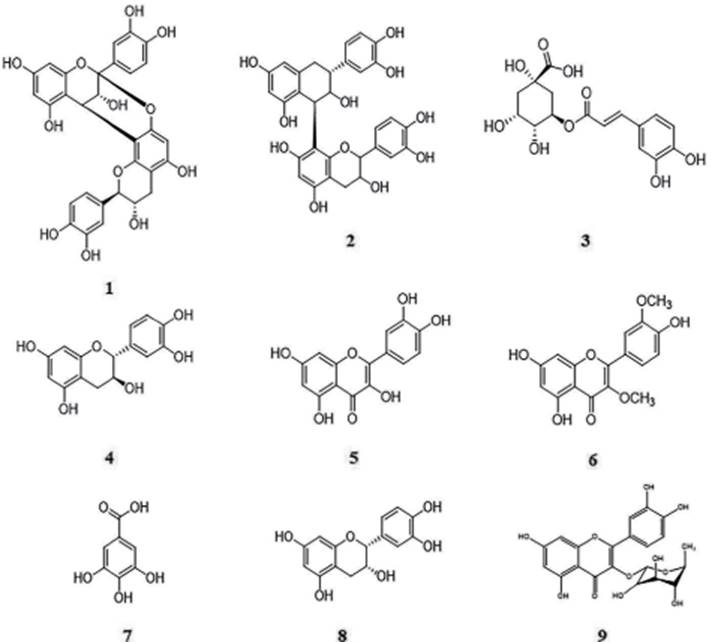
Figure 4 Chemical structures of the phenolic compounds identified in B. garcibarrigae by HPLC-ESI-MS/MS (1-8) and NMR (8-9). Procyanidin A dimer (1), procyanidin B dimer (2), chlorogenic acid (3), catechin (4), quercetin (5), quercetin-3'3-dimetthyl ether (6), gallic acid (7), epicatechin (8) and quercitrin (9).
In summary, this study showed that B. garcibarrigae bark presents potential inhibitory activities against an important enzyme related physiopathological mechanism of type 2 diabetes. It was found that the fraction FHM as a promising source of natural antioxidants, α-glucosidase inhibitors and AGEs-formation inhibitors. Thus, B. garcibarrigae has potential to be used in further investigations regarding its activity against diseases such as diabetes.