Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Obstetricia y Ginecología
Print version ISSN 0034-7434On-line version ISSN 2463-0225
Rev Colomb Obstet Ginecol vol.58 no.4 Bogotá Oct./Dec. 2007
* Visiting Fellow Maternal Fetal Medicine, Thomas Jefferson University. Philadelphia, USA. Universidad de Antioquia, Obstetrics and Gynecology Department. Correspondence: jucasemaso@gmail.com
** Second Year Fellow Maternal Fetal Medicine, Thomas Jefferson University, Philadelphia, USA. Ohio Medical College, Obstetrics and Gynecology Department.
*** Chief Maternal Fetal Medicine Department, Thomas Jefferson University, Philadelphia, USA. New York State University, Obstetrics and Gynecology Department.
RESUMEN
Una paciente de 22 años fue remitida al Hospital Universitario Thomas Jefferson de Philadelphia, Pennsylvania a las 29 semanas de embarazo, después de presentar edema generalizado y presiones arteriales elevadas. La paciente también era conocida por tener un feto con una masa sacrococcígea y polihidramnios.
En este caso se sospechó inicialmente la posibilidad de un síndrome de espejo o pseudotoxemia afectando la madre junto con una falla cardiaca en el feto. A pesar de la presencia de algunos factores que indicaban un pronóstico pobre, como son el diagnóstico de un tumor fetal en el segundo trimestre, una masa grande de crecimiento rápido, polihidramnios, hipertensión materna y un parto pretérmino, se realizó una cirugía correctiva al segundo día de vida y el resultado neonatal fue bueno.
Palabras clave: teratoma sacrococcígeo, diagnóstico prenatal, cesárea.
SUMMARY
A 22-year-old patient, having 29 weeks gestation, was referred to Thomas Jefferson University Hospital (Philadelphia, Pennsylvania) after presenting generalized oedema and high blood pressure. The patient was known to carry a foetus with a sacrococcygeal mass and polyhydramnios. A mirror syndrome affecting the mother and cardiac failure in the foetus was initially suspected. Despite some findings indicating poor prognosis for this foetus (i.e. diagnosis in the second trimester, a large rapid growing mass, polyhydramnios, maternal hypertensive disorder and preterm delivery), the neonatal outcome was good and early corrective surgery was performed on the second day of life.
Key words: sacrococcygeal teratoma, antenatal diagnosis, foetus, cesarean section.
INTRODUCTION
The incidence of congenital tumours occurring in the foetal population is very low; fetuses presenting sacrococcygeal teratoma tumours (SCT) are the most common.
Cases of foetal SCT are still rare, having an incidence calculated at 1 in 40,000 live births.1
We present a case of a foetus affected by a large mass which was rapidly growing and had an increased vascular supply. There were also some other associated factors which might have affected prognosis and were associated with poor perinatal outcome.
When and how to monitor foetal in utero wellbeing are discussed, as well as stabilising associated maternal conditions and the timing and best delivery route to improve perinatal outcome and avoid complications such as foetal cardiovascular collapse or traumatic haemorrhage prior to corrective surgery. The maternal conditions affecting the timing of delivery will also be discussed.
CASE
A 22-year-old G:1 P:0 obese, RH - non-sensitised patient was referred at 29 3/7 weeks to the Thomas Jefferson University Hospital, Philadelphia, Pennsylvania, (Jefferson is a tertiary health-care centre providing services for the metropolitan area and surrounding counties).
The patient had a history of an initial prenatal care visit at 11 weeks, followed by a normal anatomy scan at 21 weeks confirming gestational age.
A follow-up sonogram was scheduled at 26 weeks due to greater uterine size than expected data; an AFI = 32 and 8X10 cm sacrococcygeal mass were detected at that ultrasound polyhydramnios.
Progressive fluid retention and oedema were noted in the mother at 28 weeks, as well as slightly increased blood pressure readings. The 24 hour urine test revealed just 155 mg of protein.
Betametasone was used to accelerate lung maturity and the patient was then referred at 29 3/7 wks from Albert Einstein Medical Center in Philadelphia, Pennsylvania. Einstein Healthcare Network is part of Jefferson University Health System.
When admitted to Jefferson University Hospital the patient was found to have 150/100 blood pressure: 72 generalized oedema but no signs or symptoms of severe preeclampsia.
Initial labs were Hgb: 11 Hct: 35.8 platelets: 175,000, uric acid: 9.2, creatinine: 0.7, AST: 17, ALT: 25, and 24 hour urine protein of 3,322 g. The first sonographic evaluation performed with 2D/3D revealed a 1,353 g foetus having 27 AFI, consistent with polyhydramnios, and a highly vascularised sacrococcygeal solid mass (figure 1) measuring 12.2 X 8.4 X 7.5 cm (figure 2). About 90% of the mass was considered to be external to the body with only a small internal componentand broad base attachment. Some signs suggestive of early cardiac failure were also found (0.63 CC/CT ratio) suggestive of cardiomegally and positive tricuspid valve regurgitation. The umbilical artery pulsatility index (PI) was increased to 1.8 with lack of diastolic flow, suggestive of high placental resistance to blood flow; however, there was no appreciable sonographic evidence of foetal hydrops.
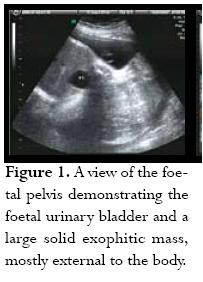
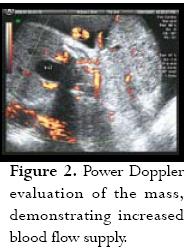
The patient remained in the hospital under close surveillance with daily NSTs and bed rest; maternal blood pressure was stable. Paediatric surgery was scheduled for opportune delivery time. One week after the patient had been admitted (30 3/7 weeks) she suffered spontaneous rupture of the membranes, which was clinically confirmed; prophylactic antibiotics were started with a combination of Ampicillin and Erythromicin. Follow-up sonogram on that day showed no evidence of cardiomegally, normal Doppler of the Ductus Venosus, middle cerebral artery and no evidence of tricuspid regurgitation. PI was again noted to be abnormally high in the umbilical artery at 1.7 with decreased diastolic flow. There was no evidence of foetal hydrops. Expectant management was proposed.
The patient had 179/96 BP, 98 °F temperature, 101 P and a decrease in foetal movements at 30 5/7 wks. NST was non-reactive. Ultrasound revealed that the foetus was in breech presentation with 2 AFI (oligohydramnios). BPP was 2/10 (non-reassuring). A decision was take to deliver the foetus 51 hours after PPROM via primary caesarean delivery using classical uterine incision. A male infant was delivered atraumatically weighing 2,190 g (40.5 cm length, 26.5 HC). The remaining amniotic fluid looked clear; there was no gross evidence of chorioamnionitis Apgar score was 8 and 9. The sacrococcygeal mass was measured to be 23 X 10 cm (figure 3). The baby was referred to DuPont Childrens Hospital for surgery in stable condition. The surgery was performed on the second day of life, without major complications. The neonates postoperative course was uncomplicated; further reconstructive surgery was planned. The baby was doing well almost 30 days post surgery; pathology report indicated a teratoma having immature components but no malignancy.
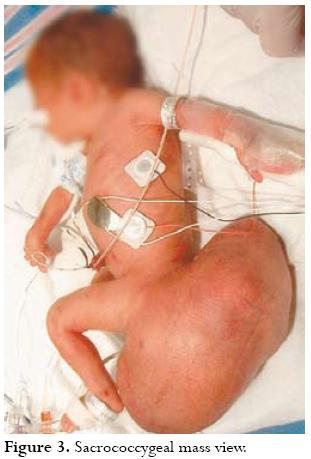
DISCUSSION
The Greek term teratos literally means monster; oma means neoplasm.2 The first description of teratoma cases were documented as far back as +- 2,000 BC.3 Incidence is rare, estimated at being 1 in 40,000 live births,1 usually having female preponderance with +- 80% of cases or a 4:1 female/male ratio.2
These tumours are believed to originate from the primitive knot or Hensens node during the second or third week of foetal development. They contain tissues derived from all three germ cell layers and can be located anywhere in the body (mediastinum, retroperitoneum, brain, etc.) but the most common location is in the sacrococcygeal area.4
The teratomas can be classified according to cell type as being mature or benign (most cases being benign, some studies reporting up to 79% of benign masses).5 Others have immature embryonic elements which may harbour a malignancy, representing the minority in the foetal population, having very low incidence or as high as 18% of cases.5
Several classification systems have been developed. Classification by consistency into solid, cystic or mixed components may be employed, the latter being most common.6 Classification by size results in small (< 5 cm) moderate (5-10) or large (> 10 cm).1
Altman et al.,1 proposed a classification based on tumour mass location and extension; however, some researchers regard such classification as useful only for description, having no prognostic value.7,8 The classification currently used by the American Academy of Pediatics Surgery Section (AAPSS) is based on the Altman classification system. Type I SCT is a completely external mass (most common), representing +- 85% of benign tumours.9 Type II SCT has both internal and external components; type III is mostly internal and type IV is a completely internal mass. The last group is the least common and seems to be associated with higher rates of malignancy ranging from 5% to 20%;9 however, this may be explained by more difficult and later detection10 at a time when malignant transformation and or metastases may have ensued.
There is also histological classification (0-3) but this cannot be performed in the uterus.
A list of SCT-associated foetal complications would include: polyhydramnios, placentomegally, foetal cardiomegally, non-immune hydrops, high-output cardiac failure, foetal bladder outlet obstruction, haemorrhage within the tumour. Other complications include malignant invasion (usually being a long-term complication). A severe maternal condition associated with SCT is Maternal Mirror Syndrome or Ballantynes Syndrome (also referred to as pseudotoxemia); this is a clinical entity presenting clinical features similar to preeclampsia in a mother carrying a foetus affected by hydrops. The mother can mimic the foetal changes and presents significant oedema; sonographic evaluation reveals hydramnios and foetal hydrops. Maternal workup is negative for preeclampsia, diabetes and cardiac or renal dysfunction. However, the mother develops symptoms similar to preeclampsia because of her hyperdynamic cardiovascular state, including vomiting, hypertension and severe peripheral oedema.11-15
In our case, the foetus showed early signs suggestive of cardiac compromise such as high umbilical artery PI with decreased diastolic component, initial cardiomegally and tricuspid regurgitation. The mother also presented signs of preeclampsia which were originally suspected to be pseudo-toxaemia or Mirror syndrome. However, Mirror syndrome was excluded due to the lack of foetal hydrops, no evidence of maternal haemodilution and elevated umbilical artery PI and lack of diastolic flow consistent with a preeclampsia. The diagnosis was then believed to be toxaemia, this being an associated finding in pregnancies affected by SCT.
Stronger predictors for poor foetal outcome are a large, solid, highly-vascularised and rapidly-growing mass with associated polyhydramnios, non-immune foetal hydrops produced by high output cardiac failure,11,16-18 this being the situation in our case and one of the reasons why the patient being admitted was kept under close foetal surveillance.
Mortality rate for foetal SCT has been shown to be as high as 50% in some studies,10 whilst others have reported a 50% prematurity rate and 33% neonatal mortality.11 A recent study reported 44.6% prematurity rate and 25% mortality16; the last results were based on a study classifying risk according to mass size.
Interestingly, the 21-week anatomy scan was apparently normal; however, such lesions may start being very small and increase their size rapidly during the third trimester. Some European studies have shown prenatal detection rates being as low as 33%.19 Prior to 1988, prenatal detection rates were only 11%; detection rates have since increased to 53% in more recent studies. These numbers may seem surprisingly low for medical personnel dedicated to prenatal diagnosis but many factors must be taken into consideration, such as experience, available technology, gestational age at the time of scanning, a patients body habitus, foetal position, etc. Many articles now describe the role of volume ultrasound (3-D) in helping to make or confirm a diagnosis.9
SCT should be considered in differential diagnosis in any pregnancy having an unexplained high level of maternal serum AFP, signs of foetal cardiac failure, polyhydramnios and/or a sacral mass. Careful foetal anatomical evaluation needs to be made in view of the possible presence of a foetal tumour.
When diagnosis is already obvious because of the presence of a large mass, complications are not just limited to foetal cardiac failure with hydrops but the possibility of polyhydramnios and the associated risk of preterm labour. In this pregnancy, polyhydramnios was probably the main factor producing an early rupture of membranes. During labour there is also a high possibility of dystocia and even foetal death due to trauma to the mass precipitating haemorrhage within the tumour (resulting in around 9% mortality rate).1,20
In utero foetal surgery is recommended only for < 30 week foetuses having clear signs of advanced cardiac failure or hydrops. The use of betamethasone is indicated when an early delivery is to be expected. Expectant management is also indicated if there is no evidence of maternal compromise such as Mirror syndrome or preeclampsia or foetal cardiovascular compromise. Delivery is indicated once foetal lung maturity has been established by amniocentesis.21 Cesarean section delivery is recommended for all babies having a mass bigger than 5 cm.20
For masses as large as that in this case (> 10 cm), having a high risk of bleeding, a cesarean section via classical uterine incision is advised to facilitate atraumatic delivery.16 In our case, the presence of a baby in a breech presentation with associated oligohydramnios, a large tumour mass, non-reactive NST and low BPP led to taking the decision to deliver the baby following the above type of procedure to avoid foetal demise in utero and minimise trauma to the mass which could have jeopardised post-delivery survival.
Authors note: the protected health information (PHI) collected from the patients clinical chart and counselling interviews were carefully deidentified and modified in compliance with the Health Insurance Portability and Accountability Act (HIPAA) privacy rule.
REFERENCES
1. Altman RP, Randolph JG, Lilly JR. Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J Pediatr Surg 1974;9:389-98. [ Links ]
2. Arceci RJ, Weinstein HJ. Neoplasia. In: Avery GB, Fletcher MA, McDonald ME. Neonatology, pathophysiology and management of newborn. Philadelphia: Lippincott; 1994. p.1219-20. [ Links ]
3. Ballantine JW. Teratologie. Williams and Nougate; 1894. [ Links ]
4. Tuladhar R, Patole SK, Whitehall JS. Sacrococcygeal teratoma in the perinatal period. Postgrad Med J 2000;76:754-9. [ Links ]
5. Gabra HO, Jesudason EC, McDowell HP, Pizer BL, Losty PD Sacrococcygeal teratoma-- a 25-year experience in a UK regional center. J Pediatr Surg 2006;41:1513-6. [ Links ]
6. Keslar PJ,Buck JL, Suarez ES. Germ cell tumors of sacrococcygeal region: radiologic pathologic correlation. Radiographics 1994;14:607-22. [ Links ]
7. Bilik R, Shandling B, Pope M, Thorner P, Weitzman S, Ein SH, et al. Malignant benign neonatal sacrococcygeal teratoma. J Pediatr Surg 1993;28:1158-60. [ Links ]
8. Schmidt B, Haberlik A, Uray E, Ratschek M, Lackner H, Höllwarth ME. Sacrococcygeal teratoma: clinical course and prognosis with a special view to long-term functional results. Pediatr Surg Int 1999;15:573-6. [ Links ]
9. Bonilia-Musoles F, Machado LE, Raga F, Osborne NG, Bonilla F Jr. Prenatal diagnosis of sacrococcygeal teratomas by two and three dimensional ultrasound. Ultrasound Obstet Gynecol 2002;19:200-5. [ Links ]
10. Bianchi DW, Cromblehome TM, Dalton ME. Fetology. Diagnosis and management of the fetal patient. In: Sacrococcygeal teratoma. Chapter 116. Mc Graw-Hill; 2000. [ Links ]
11. Holterman AX, Filiartrault D, Lallier M, Youssef S. The natural history of Sacrococcygeal teratomas diagnosed through routine obstetric sonogram a single institution experience. J Pediatr Surg 1998;33:899-903. [ Links ]
12. Roberts JM, Taylor RN, Musu TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 1989;161:1200-5. [ Links ]
13. van Selm M, Kanhai HH, Gravenhorst JB. Maternal hydrops syndrome: a review. Obstet Gynecol Surv 1991;46:785-8. [ Links ]
14. Vidaeff AC, Pschirrer ER, Mastrobattista JM, Gilstrap LC, Ramin SM. Mirror syndrome. A case report. J Reprod.Med 2002;47:770-4. [ Links ]
15. Heyborne KD, Chism DM. Reversal of Ballantyne syndrome by selective second trimester fetal termination: a case report. J Reprod Med 2000;45:360-2. [ Links ]
16. Benachi A, Durin L, Maurers SV, Aubry M, Parat S, Herlicoviez M, et al. Prenatally diagnosed sacrococcygeal teratoma: a prognostic classification. J Pediatr Surg 2006;41:1517-21. [ Links ]
17. Perreli L, Durzo C, Manzoni C, Pintus C, De Santis M, Masini L, et al. Sacrococcygeal teratoma. Outcome and management. An analysis of 17 cases. J Perinat Med 2002;30:179-84. [ Links ]
18. Westerburg B, Feldstein VA, Sandberg PL, Lopoo JB, Harrison MR, Albanese CT. Sonographic prognostic factors in fetuses with sacrococcygeal teratoma. J Pediatr Surg 2000;35:322-5. [ Links ]
19. De BackerA, Erpicum P, Philippe P, Demarche M, Otte JB, Scwagten K, et al. Sacrococcygeal teratoma: results of a retrospective multicentric study in Belgium and Luxembourg. Eur J Pediatr surg 2001;11:182-5. [ Links ]
20. Chervenak FA, Isaacson G, Touloukian R, Tortora M, Berkowitz RL, Hobbins JC. Diagnosis and management of fetal teratomas. Obstet Gynecol 1985;66:666-71. [ Links ]
21. Adzick NS, Crombelhome TM, Morgan MA, Quinn TM. A rapidly growing foetal teratoma. Lancet 1997;349:538. [ Links ]
Conflict of interests: none.














