INTRODUCTION
Pre-term delivery is defined as that which occurs before 37 weeks of gestation, and it is one of the main causes of infant morbidity and mortality and morbidity in adult life 1-5, and it accounts for close to 70% of perinatal mortality. Every year, 15 million premature neonates are born and, of them, 1.1 million die as a result of organic and functional immaturity 6,7, with the resulting implications familial, societal and economic implications. Annual expenditures associated with care in the United States are estimated at approximately 25 billion dollars 6. Frequency varies, and it is higher in medium and low income countries. The global prevalence is estimated at 9.6% and, by regions, it is 8.1% in Latin America and the Caribbean, 6.2% in Europe, and 10.6% in North America. In Colombia, frequency ranges between 10 and 12% 7. One of the causes is chorioamnionitis 8-10, and it is important to distinguish between clinical chorioamnionitis (CC) and histologic chorioamnionitis (HC). In CC, diagnosis is usually clinical, based on the criteria proposed by Gibbs 11,12; in the less studied HC, there is an acute inflammatory process characterised by neutrophil infiltration in each of the placental structures. When both chorion and amnion are involved, it is called acute chorioamnionitis; when it affects the villi, it is called acute villitis; if the process involves the umbilical cord, it is called funisitis 13. In the foetus, the counterpart condition is foetal inflammatory response syndrome 13,14.
The word "histologic" has been introduced in order to make the specific distinction between the clinical syndrome (CC) and the pathologic diagnosis (HC). The terms are not synonymous and they give rise to confusion when used interchangeably 13. On the other hand, the diagnosis of HC is significant considering that it has been found to be a risk factor for adverse perinatal outcomes, including even cerebral palsy 10,15-18. The prevalence of HC is related to gestational age (GA): the lower the GA, the higher the prevalence 9. Prevalence has been described to be as high as 94.4% in foetuses under 24 weeks 19, although this is controversial.
The antepartum test of choice for chorioamnionitis is amniocentesis for amniotic fluid (AF) culture testing, despite it being an invasive, non-harmless test 20. AF Gram staining 21, glucose concentration, lactic dehydrogenase (LDH) levels and leukocyte stearase levels are used to assess for infection in the amniotic cavity, because of their low cost and ready availability 22-25. Another marker is C reactive protein (CRP) which, although not included in Gibbs' criteria, has gained great importance for diagnosis 26,27. Ultrasound markers have been described, including cervical shortening, the presence of sludge, and the foetal biophysical profile (FBP) 28, the latter being a good predictor of infection when the time interval between its performance and delivery is < 24 hours 29-31. The focus of most research into markers has been diagnostic yield for CC, and there are less studies focusing on HC.
In view of the shortage of data regarding the prevalence of HC in our setting, and considering the behaviour of clinical and paraclinical parameters in HC, this study was designed with the goal of determining the prevalence of HC in patients with spontaneous preterm labour seen at the San José University Hospital (HUSJ) in Popayán, and of identifying potential social and demographic risk factors and the association with clinical and paraclinical signs.
MATERIALS AND METHODS
A descriptive prevalence study was conducted with pregnant women presenting with spontaneous preterm delivery before 36 weeks and 6 days, with a singleton live neonate, from whom placentas were available. The study was conduced between July 1, 2014 and May 16, 2016, at a public high complexity referral centre for the Department of Cauca and southwestern Colombia that receives patients covered by both the contributive and subsidised healthcare regimes. Cases of secondary preterm deliveries and major congenital malformations were excluded. For sample size purposes, the formula n = (p*q)/ (E/1,96)2 was used, with a minimum expected prevalence of 12% (9, 16), a tolerated margin of error of 5%, a 95% confidence interval, non-response adjustment of 10%, correction for finite population and a population base of 1900 births during the year prior to the study, for a final sample size of 166 pregnant women. Consecutive non-probabilistic sampling was used.
Procedure. Women with a diagnosis of spontaneous preterm delivery estimated on the basis of the first trimester ultrasound or of the physical examination of the neonate by the paediatrician at the time of birth were asked for their informed consent to participate in the study. Following authorisation to participate in the study, placentas were retrieved and placed in a plastic container with 10% buffer in a proportion of 1:20. Each container was duly identified and transferred on the same day to the HUSJ pathology department where the following protocol for placenta processing was applied: after assigning an identification code, the placenta was examined starting with the membranes, followed by the cord and finally the foetal and maternal surfaces. Cross-sectional cuts were made of the cord and placenta, taking 2-3 cm sections from the start of the membranes at the point where they rupture to the placental margin. Following a 24-hour fixation period and post-fixation and cutting processes, the sections were stained with haematoxylin and eosin and then given to the pathologist for interpretation. The pathologist had no knowledge of the reason why the placental study was requested. The diagnosis of HC was based on the identification of maternal polymorphonuclear leukocytes in the ovular membranes, the chorionic plate and the cord 13,32,33.
After placenta retrieval, the patients were interviewed by trained personnel using a semi-structured questionnaire designed by the researchers, reviewed by experts and pilot-tested. Information was also taken from the institutional clinical record. A database was created in Excel using validation rules for entry control in order to ensure data reliability and quality; the analysis was later performed using the Stata version 10.0 software package.
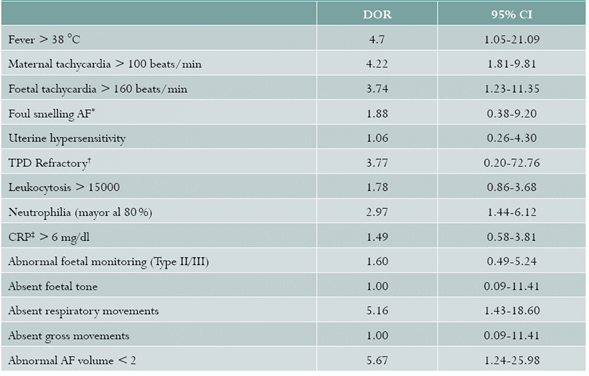
The following variables were measured: maternal age; maternal age stratified as > and< than 34 weeks because, as prevalence studies reveal, HC is higher the lower the GA 15,16,34-39; area of residence; type of union; race (white and mestizo as reference and black and indigenous as risk); type of health coverage (contributive or subsidised); socioeconomic bracket (brackets 3, 4 and 5 as reference, and 1 and 2 as risk); adequate prenatal care (number of visits > 3) and GA at the start of follow-up during the first trimester; obstetric formula; a history of preterm labour; urinary tract infection diagnosed by urine culture; number of vaginal examinations (> 6 as risk); refractory uterine activity (progression of delivery despite management). The following diagnostic findings were assessed: fever (> 38 °C), maternal tachycardia (> 100 beats/min), foetal tachycardia (> 160 beats/ min), abnormal CRP (> 6 mg/dl), WBC (leukocytes > 15000 and neutrophils > 80% as abnormal), AF characteristics (fetid and non-fetid), foetal biophysical profile (FBP) analysing each of the components (tone, AF volume, respiratory and gross movements), foetal monitoring (category I as normal and categories II and III as abnormal).
Statistical analysis. In order to determine the prevalence of HC, the population with a positive histopathological study was used as the numerator, and the total number of pregnant women with spontaneous preterm delivery and available histopathology was used as the denominator. Variables were analysed individually, from an exploratory perspective, in order to consider distribution normality and identify extreme and lost values that could influence the result; then, the group of pregnant women with preterm delivery and HC was compared with the group with preterm delivery but no HC. The prevalence ratio (PR) was used as the association measure with its 95% confidence interval (CI) in order to determine the frequency of exposure in the groups with and without HC. Additionally, diagnostic odds ratio (DOR) was determined for paraclinical parameters and their respective 95% CI. The Student t test was used for continuous variables with a normal distribution folowing a variance analysis. The Mann-Whitney U test was used for non-normal distribution following the application of the Shapiro Wilk normality test and the chi square or the Fisher test, as relevant.
The study was endorsed by the Ethics Committee of the HUSJ. Information confidentiality was ensured.
RESULTS
During the study period, care was provided for a total of 3,385 births (July-December, 2014, 882; January-December 2015, 1,714; and January-May 2016, 789). Of these, 172 (5.1%) were spontaneous preterm deliveries; no placentas were available for 12 cases that were excluded, leaving 160 for analysis. Of these 160 placentas, 100 were found to have HC, for a prevalence of 68.75% (95% CI: 61.49-76.00).
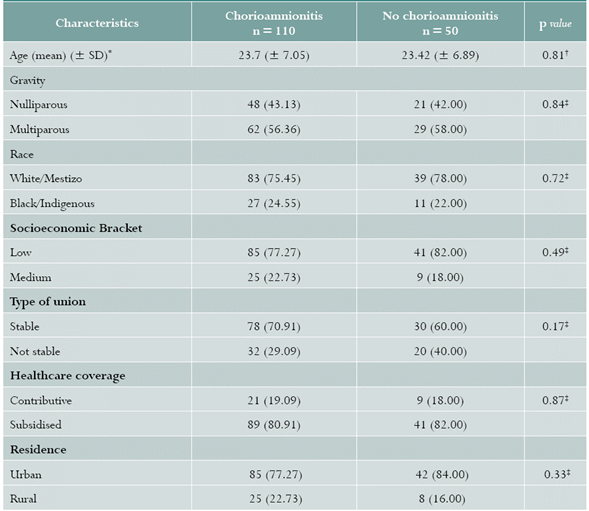
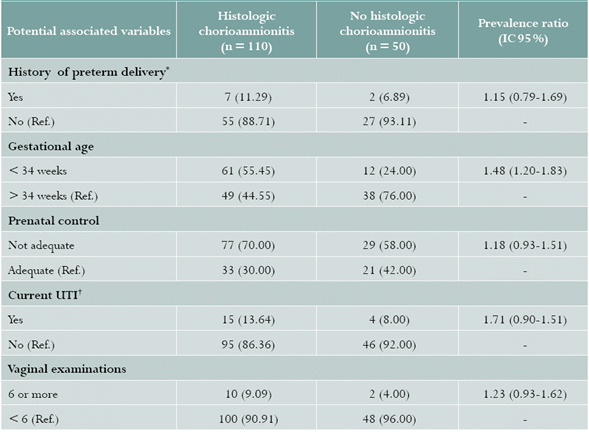
No differences were found regarding baseline characteristics, maternal age, gravity, and social and demographic characteristics (Table 1). As for clinical history, HC was more prevalent in the GA group of < 34 weeks (PR = 1.48; 95% CI: 1.20-1.83); for the remaining variables, similar PRs were found between the groups (Table 2).
Table 1 Baseline characteristics of pregnant women with preterm delivery at HUSJ, Popayán, Colombia. July 2014-May 2016
Source: study data
Values are expressed in (%)
* SD: standard deviation
† Student t test
‡ Chi square
Table 2 Prevalence ratios of histologic chorioamnionitis. HUSJ, Popayán, Colombia. July 2014-May 2016
Source: study data.
Values expressed in (%)
* Nulliparous patients were excluded
† UTI: urinary tract infection
Regarding associations of signs and symptoms, a significant association was found with fever, maternal tachycardia, foetal tachycardia, absent respiratory sounds, AF volume lower than 2, and presence of neutrophilia (Table 3).
DISCUSSION
Our study found a prevalence of 68% for HC. Association was found with gestational age under 34 weeks and with general maternal symptoms, diminished respiratory movements in the biophysical profile, a larger AF volume on obstetric ultrasound, and neutrophilia.
As far as prevalence is concerned, our findings are consistent with research published by Xie et al., who found a frequency of 68.0% of HC in pregnancies between 28 and 33 weeks + 6 days of gestation (138/203) 15. Xie et al., in 371 gestations under 34 weeks, reported a prevalence of 70% 16; Holzman et al. report a prevalence of 63% 34, as is also the case with Nowak et al., who show a prevalence of HC of 73.7% in gestations of less than 35 weeks 35. In contrast, other authors report lower prevalences, like the study by Lee et al., that reports a prevalence of 24% in preterm deliveries between weeks 34 and 36 + 6 days 10; Romero et al. show that the prevalence of HC is given as a function of GA at birth 9; Xie et al. found that the incidence of HC varied according to GA: between weeks 28 and 29, 86.7%; between weeks 30 and 31, 70.1%; and between weeks 32 and 33, 61.4% 16.
Regarding diagnostic criteria, Curtin et al., in a retrospective cohort, report the presence of fever in 80.6% of patients with HC (OR = 4.63; 95% CI 3.09-6.93), and conclude that fever is an independent predictor of HC 40; and in a different retrospective cohort with 641 placentas examined, they report, for fever, an OR = 2.83 (95% CI: 1.76-4.55) 41.Heller et al. did not find significant differences in the proportion of intrapartum fever between the groups with and without HC 42. When analysing foetal wellbeing variables, Curtin et al. report that no association was found, using a multivariate analysis, between severe variable decelerations and HC 41, while other authors do report that relationship. Salafia et al. found a direct relationship between severe variable decelerations of foetal heart rate (FHR) antepartum and HC (p = 0.012), and also, during labour, reduced variability of FHR was associated with HC (p = 0.005) 43. Regarding the absence of respiratory movements, Sherer et al.,in preterm deliveries under 32 weeks, reported a higher frequency (32%) of absent respiratory movements in severe umbilical vasculitis as compared to 15%, this being a significant finding (p = 0.049) 44; Ghidini et al., in gestations under 32 weeks, did not find a relationship between acute placental inflammation and the final FBP score or its individual components 45.
The following are the strengths of this study: the outcome variable was standardised, pathologists were not aware of the suspected diagnosis, GA was reliable, and the parameters considered for the analysis were the closest to the time of birth.
Weaknesses include the potential reference bias considering that the study was conducted in a high complexity institution where patient population is of a higher obstetric risk; moreover, there were 12 losses (7%) for which there were no placentas available for study, giving rise to a potential underestimation of prevalence. Additionally, one of the variables included was urinary tract infection which, in itself, might explain some of the clinical and paraclinical findings.











 text in
text in